Interpreting The JUPITER Trial: Statins can prevent VTE, but more study is needed
ABSTRACTAnalysis of a secondary end point of the JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) found that a statin reduced the risk of venous thromboembolism (VTE) in apparently healthy people with high levels of C-reactive protein and normal levels of low-density lipoprotein cholesterol (N Engl J Med 2009; 360:1851– 1861). Still, pending more study, statins should not be substituted for proven prophylaxis and anticoagulation, especially for patients with recurrent deep venous thrombosis, hospitalized patients, postoperative patients, and other patients prone to VTE.
KEY POINTS
- Risk factors for VTE overlap with those for arterial thrombosis, although the data are mixed.
- The statin drugs have a number of effects on factors other than lipid levels, notably on markers of inflammation and on clotting factors.
- In the JUPITER trial, the incidence of VTE in people taking rosuvastatin (Crestor) 20 mg/day was about half that in people taking placebo. This was a relatively healthy population, and the incidence in both groups was low.
- Further study is needed in patients at risk of VTE.
A major placebo-controlled trial has found that a statin can reduce the risk of venous thromboembolism (VTE).1
We do not recommend prescribing this class of drugs for this purpose until much more research has been done, and we certainly do not recommend substituting a statin for anticoagulant therapy in a patient at risk of VTE.
Nevertheless, we are excited by the latest findings, and we find comfort in knowing that if a patient is taking a statin for an approved indication, ie, reducing the risk of cardiovascular disease in a patient with hyperlipidemia or a previous cardiovascular event, the drug will also reduce the risk of VTE.
In the pages that follow, we describe and comment on what is known about the effect of statins on the risk of VTE.
ARTERIAL AND VENOUS THROMBOSIS: HOW ARE THEY LINKED?
The causes of arterial thrombosis may not be entirely distinct from those of deep vein thrombosis and pulmonary embolism, collectively referred to as VTE. Some studies have found that risk factors for arterial thrombosis overlap with those for VTE.2–4 However, other studies have shown no association between venous and arterial events.5–10
Hyperlipidemia, in particular, has been evaluated to see if it is a risk factor for VTE. As with other risk factors for arterial thrombosis, the data have been mixed, with some reports favoring an association with VTE and others not.4,5,11 Even so, preventive strategies targeting arterial risk factors have shown promise in reducing VTE events.12
Although commonly used to treat hyperlipidemia, statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) are believed to reduce the incidence of thrombosis by a number of mechanisms13:
- Decreasing platelet aggregation
- Inhibiting expression of tissue factor and plasminogen activator inhibitor 1
- Increasing expression of tissue plasminogen activator
- Increasing expression of thrombomodulin, which can activate protein C and prevent thrombin-induced platelet and factor V activation and fibrinogen clotting.
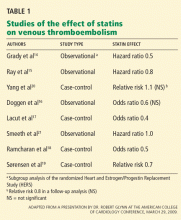
STATINS AND VTE IN OBSERVATIONAL AND CASE-CONTROL STUDIES
In view of the multiple effects of statins, several studies have looked at whether these drugs reduce the occurrence of both arterial thrombosis and VTE.14–19
Two prospective observational studies and four case-control studies found that statins reduced the risk of VTE by 20% to 60%.14–19 Interestingly, two of the case-control studies found that antiplatelet therapy did not reduce the risk of VTE.18,19
THE JUPITER STUDY
The Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) study primarily sought to determine if rosuvastatin (Crestor) 20 mg/day, compared with placebo, would reduce the rate of first major cardiovascular events.22 A prespecified secondary end point of the trial was VTE, making JUPITER the first randomized, placebo-controlled trial to specifically test whether statins prevent VTE.1
Inclusion criteria: Normal LDL, high CRP
The study included men age 50 and older and women age 60 and older with no history of cardiovascular disease. In addition, their lowdensity lipoprotein (LDL) cholesterol levels had to be lower than 130 mg/dL (3.4 mmol/L), their triglyceride levels had to be lower than 500 mg/dL (5.6 mmol/L), and their highsensitivity C-reactive protein (hs-CRP) levels had to be 2.0 mg/L or higher.
Since high levels of hs-CRP, a marker of inflammation, predict cardiovascular events and since statins lower hs-CRP levels, the investigators hypothesized that people with elevated hs-CRP but without hyperlipidemia might benefit from statin treatment.21
Patients were excluded if they had received lipid-lowering therapy within 6 weeks of the trial screening, had diabetes mellitus or uncontrolled hypertension, were currently using postmenopausal hormone-replacement therapy, or had had cancer within the previous 5 years, except for certain skin cancers.
Candidates who complied well during a 4-week placebo run-in phase were randomly assigned to receive either rosuvastatin 20 mg daily (an intermediate dose) or a matching placebo. In all, 17,802 people were randomized. The two assigned groups appeared to be well matched.
Patients were to come in for visits twice a year for 60 months after randomization to be assessed for symptomatic deep venous thrombosis and pulmonary embolism. New cases of VTE were confirmed by imaging studies, by the initiation of anticoagulation therapy, or by death ascribed to pulmonary embolism.
Idiopathic VTE was classified as unprovoked if it occurred in the absence of trauma, hospitalization, or surgery within 3 months before the event, and in the absence of any diagnosed cancer within 3 months before and after the event. Provoked VTE events were those that occurred in a participant with cancer or when a precipitating event was associated with trauma, hospitalization, or surgery.