Managing acute upper GI bleeding, preventing recurrences
ABSTRACTAcute upper gastrointestinal (GI) bleeding is common and potentially life-threatening and needs a prompt assessment and aggressive medical management. All patients need to undergo endoscopy to diagnose, assess, and possibly treat any underlying lesion. In addition, patients found to have bleeding ulcers should receive a proton pump inhibitor, the dosage and duration of treatment depending on the endoscopic findings and clinical factors.
KEY POINTS
- The first priority is to ensure that the patient is hemodynamically stable, which often requires admission to the intensive care unit for monitoring and fluid resuscitation.
- Peptic ulcers account for most cases of upper GI bleeding, but bleeding from varices has a much higher case-fatality rate and always demands aggressive treatment.
- Patients with ulcer disease should be tested and treated for Helicobacter pylori infection.
- Patients with a history of bleeding ulcers who need long-term treatment with aspirin or a nonsteroidal anti-inflammatory drug should also be prescribed a proton pump inhibitor.
Endoscopy to diagnose bleeding and assess risk
Upper endoscopy is 90% to 95% diagnostic for acute upper GI bleeding.36
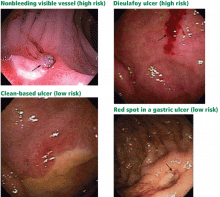
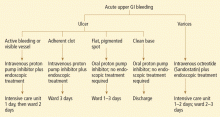
Patients at high risk (ie, older than 60 years, with severe comorbidity, or hemodynamically compromised) who have active bleeding (ie, witnessed hematemesis, red blood per nasogastric tube, or fresh blood per rectum) or a nonbleeding visible vessel should be admitted to a monitored bed or intensive care unit. Observation in a regular medical ward is appropriate for high-risk patients found to have an adherent clot. Patients with low-risk findings (eg, a clean ulcer base) are at low risk of recurrent bleeding and may be considered for early hospital discharge with appropriate outpatient follow-up.
Endoscopy to treat bleeding
About 25% of endoscopic procedures performed for upper GI bleeding include some type of treatment,39 such as injections of epinephrine, normal saline, or sclerosants; thermal cautery; argon plasma coagulation; electrocautery; or application of clips or bands. They are all equally effective, and combinations of these therapies are more effective than when they are used individually. A recent meta-analysis found dual therapy to be superior to epinephrine monotherapy in preventing recurrent bleeding, need for surgery, and death.40
How to manage adherent clots is controversial, but recent studies have revealed a significant benefit from removing them and treating the underlying lesions compared with drug therapy alone.43,45
Flat, pigmented spots and nonbleeding ulcers with a clean base do not require endoscopic treatment because the risk of recurrent bleeding is low.
Endoscopic therapy stops the bleeding in more than 90% of patients, but bleeding recurs after endoscopic therapy in 10% to 25%.46 Reversal of any severe coagulopathy with transfusions of platelets or fresh frozen plasma is essential for endoscopic hemostasis. However, coagulopathy at the time of initial bleeding and endoscopy does not appear to be associated with higher rates of recurrent bleeding following endoscopic therapy for nonvariceal upper GI bleeding.47
Patients with refractory bleeding are candidates for angiography or surgery. However, even when endoscopic hemostasis fails, endoscopy is important before angiography or surgery to pinpoint the site of bleeding and diagnose the cause.
A second endoscopic procedure is generally not recommended within 24 hours after the initial procedure.48 However, it is appropriate in cases in which clinical signs indicate recurrent bleeding or if hemostasis during the initial procedure is questionable. A meta-analysis found that routinely repeating endoscopy reduces the rate of recurrent bleeding but not the need for surgery or the risk of death.49
ALL PATIENTS SHOULD BE ADMITTED
VARICEAL BLEEDING
Variceal bleeding, a severe outcome of portal hypertension secondary to cirrhosis, carries a 6-week mortality rate of 10% to 20%.50 In view of the risk, primary prevention is indicated in patients with high-risk varices.
The mainstays of primary and secondary prevention are the nonselective beta-blockers such as nadolol (Corgard) and propranolol (Inderal). Several randomized controlled trials have shown lower rates of recurrent bleeding and death with propranolol or nadolol than with placebo.51 In doses that decrease the heart rate by 25%, beta-blockers have been shown to delay and decrease variceal hemorrhage. However, most patients require prophylactic endoscopic variceal ligation because they cannot tolerate beta-blocker therapy.
In suspected acute variceal bleeding, a somatostatin analogue should be started to decrease the portal pressure, and antibiotics should be started to reduce the risks of infection and death. Vasoactive drugs, ie, somatostatin analogues, should be started before endoscopy and continued for 5 days to reduce the chances of recurrent bleeding.52,53
Terlipressin is the only drug proven to improve the odds of survival in acute variceal bleeding. Although widely used in Europe, it has not been approved for use in the United States.
Octreotide, another option, improves hemostasis to the same extent, although it does not increase the survival rate.54,55 The recommended dose of octreotide for patients with variceal bleeding is a 50-μg intravenous bolus, followed by a 50-μg/hour infusion for 5 days.
Combining endoscopic and drug therapy improves the chances of stopping the bleeding and reduces the risk of recurrent bleeding compared with endoscopic therapy alone.56
Transjugular intrahepatic portosystemic shunting is indicated in recurrent variceal hemorrhage or in those with initial bleeding that is refractory to standard medical and endoscopic therapy. It is not the primary therapy because it doubles the risk of encephalopathy and has a high stent occlusion rate (up to 60%, lower with covered stents).