The homocysteine hypothesis: Still relevant to the prevention and treatment of cardiovascular disease?
ABSTRACTAlthough evidence suggests that the homocysteine hypothesis is still relevant as a predictor of cardiovascular risk, we cannot conclude that measuring the homocysteine level is useful in guiding treatment. Furthermore, studies of primary and secondary prevention show no evidence that taking folic acid or other B vitamins lowers the risk of cardiovascular events.
KEY POINTS
- Factors that can cause the plasma homocysteine concentration to be high include deficiencies of vitamin B6, vitamin B12, and folic acid; renal insufficiency; and genetic variants in enzymes responsible for homocysteine metabolism.
- Higher plasma homocysteine levels are associated with a higher risk of cardiovascular, cerebrovascular, and peripheral arterial disease.
- Supplementation of B vitamins and folic acid can lower plasma homocysteine levels.
- Randomized controlled trials of supplementation to prevent cardiovascular events and other adverse outcomes have had mostly negative results. However, most patients in these trials had normal baseline plasma homocysteine levels.
- Needed are randomized trials to see if supplementation improves outcomes in patients with high homocysteine levels.
Patients often ask primary care physicians and cardiologists about the measurement of biomarkers for cardiovascular disease and about the efficacy of preventive measures.
Although studies have shown that elevated homocysteine is a risk factor for cardiovascular and peripheral arterial disease1–3 and that supplementation with folic acid, vitamin B6, and vitamin B12 lowers homocysteine levels,4,5 it is unclear whether such supplementation prevents cardiovascular events. As a result, there is no consensus about whose homocysteine levels should be measured and who, if anyone, should receive homocysteine-lowering therapies.
The aim of this paper is to examine whether the evidence is sufficient to recommend homocysteine testing to guide the prevention and treatment of cardiovascular disease, or to recommend using folic acid, vitamin B6, and vitamin B12 for primary or secondary prevention of cardiovascular disease.
HISTORY OF HOMOCYSTEINE AS A RISK MARKER
Homocysteine is an amino acid formed from the metabolism of methionine, an essential amino acid derived from dietary protein. Although homocysteine was first isolated by Butz and du Vigneaud in 1932,6 it was not until 1964 that Gibson et al7 reported that patients with homocystinuria (more about this below) had vascular anomalies and arterial thrombosis. In 1969, McCully8 made the connection between elevated homocysteine levels and the risk of atherosclerosis.
Several possible mechanisms for the association between homocysteine and atherosclerosis have been demonstrated in experimental models. These include stimulation of smooth muscle growth, reduction in endothelial cell growth, impaired endothelial cell relaxation, decreased synthesis of high-density lipoprotein, promotion of autoimmune response, and accumulation of inflammatory monocytes in atherosclerotic plaques.3,9,10
In view of these findings, researchers have been evaluating whether homocysteine-lowering therapies decrease the risk of cardiovascular disease.
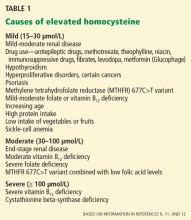
CAUSES OF ELEVATED PLASMA HOMOCYSTEINE
Vitamin deficiencies. Vitamin B6 (pyridoxine), vitamin B12 (cyanocobalamin), and folic acid are cofactors required for homocysteine metabolism, and deficiency in any or all of these leads to disruption of the relevant metabolic pathways.
Renal impairment. A low glomerular filtration rate has also been correlated with an elevated plasma homocysteine concentration. This makes sense, since the kidneys perform up to 70% of the clearance of homocysteine, although a cause-and-effect relationship is unclear.13
Inborn errors of homocysteine metabolism.Homocystinuria, ie, an abnormal elevation of homocysteine in the urine, is caused by several autosomal recessive disorders. People with these genetic variations have extremely high homocysteine levels.
A deficiency in the enzyme cystathionine beta-synthase is quite rare (the incidence in newborn babies has been found to be 1 in 344,000 worldwide and 1 in 65,000 in Ireland and Australia14), but leads to homocysteine levels greater than 100 μmol/L and often causes cardiovascular disease by the age of 30 years.15
A deficiency in the enzyme methylene tetrahydrofolate reductase (MTHFR) is a more common cause of mildly to moderately elevated plasma homocysteine levels.16 The MTHFR deficiency involves a variation at position 677 in the MTHFR gene in which cytosine is replaced by thymidine (thus called C677T or 677C>T).17 Ten percent of the population are homozygous for this variant (TT), 43% are heterozygous (CT), and 47% are unaffected (CC). Heterozygotes have slightly higher homocysteine levels than unaffected people, while people with the TT genotype have approximately 20% higher homocysteine levels.17
ELEVATED HOMOCYSTEINE IS COMMON
In a study of a population in Norway from 1992 to 1993, 8.5% had mild elevations in homocysteine (plasma levels 15–29.99 μmol/L), 0.8% had moderate elevations (30–99.99 μmol/L), and 0.02% had severe elevations (≥ 100 μmol/L).13,18 The prevalence of hyperhomocysteinemia in the United States is probably much lower, given that supplementation of white flour and cereal grains with folic acid has been mandatory since 1998, but this is not well described in the literature.