Intracerebral hemorrhage: Pick your poison
Anticoagulants have been helping patients at risk of thrombosis since the late 1930s.1,2 Although the indications for these agents are many, the development of anticoagulants beyond oral vitamin K antagonists and parenteral heparin has been slow. In the United States, the vitamin K antagonist warfarin (Coumadin) is still the only oral anticoagulant available.
The major complication of anticoagulant therapy is bleeding, and vitamin K antagonists have proven challenging to use in clinical practice.1,3 They have a narrow therapeutic window, they vary considerably in dose-response from patient to patient, and they are subject to significant interactions with other drugs and with foods. For these reasons, therapy must be monitored with laboratory testing, and good patient compliance and patient education are essential. Yet even with these measures, life-threatening hemorrhage still can occur.
In this issue of the Cleveland Clinic Journal of Medicine, Goldstein and Greenberg4 review warfarin-related intracerebral hemorrhage (ICH) and provide a framework for considering whether to resume anticoagulant therapy.
WHAT TO DO IN THE ACUTE PHASE
Goldstein and Greenberg divide the difficult clinical question of what to do after ICH into the acute phase and the chronic phase.
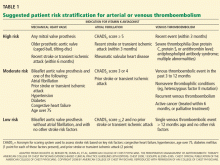
What to do in the acute phase appears straightforward, as the risk of hematoma expansion in the hours immediately after warfarin-related ICH outweighs the risk of arterial or venous thromboembolism. Anticoagulant reversal should be the primary consideration in the first 24 hours, and, assuming the patient does not have acute (< 4-week-old) deep vein thrombosis, intermittent pneumatic compression should be applied to the lower extremities to reduce the risk of venous thromboembolism associated with ICH.5
Prophylactic anticoagulation with subcutaneous fixed-dose heparin or low-molecular-weight heparin is recommended starting 72 hours after ICH is diagnosed, provided the patient is not underweight (< 50 kg), has relatively normal renal function (creatinine clearance > 30 mL/minute/1.73 m2) and normal platelet function, and does not have coagulopathy. 6 If any one of these criteria is not met, the risk of bleeding can be higher, even with only prophylactic doses of anticoagulant drugs. Prophylactic anticoagulation should be continued until hospital and rehabilitation discharge, typically 1 to 2 weeks after ICH, depending on the severity of the patient’s neurologic impairment.
If a patient with warfarin-related ICH has concomitant acute proximal deep vein thrombosis or pulmonary embolism (ie, < 4 weeks old), then caval interruption therapy would be indicated.7 Although retrievable inferior vena cava filters are increasingly preferred over permanent filters, it is important to recognize the relative lack of both longitudinal and prospective data on retrievable devices. Given that provoked venous thromboembolism requires a minimum of 3 months of anticoagulation, and retrievable filters generally need to be removed before 3 months, a retrievable filter should be chosen only if the clinician has already decided that oral anticoagulation will be restarted in the next 3 to 4 weeks after filter removal.
WHAT TO DO IN THE CHRONIC PHASE
A more difficult question in patients with warfarin-related ICH arises in the chronic phase: should oral anticoagulation be resumed at all?
Since the risk of ICH is related to the intensity of anticoagulation, a lower target international normalized ratio may be the best compromise, depending on the patient. Alternatively, antiplatelet therapy alone may offer some benefit with less risk of ICH.