Acetaminophen: Old drug, new warnings
ABSTRACT
The US Food and Drug Administration (FDA), concerned about the incidence of acute liver failure due to acetaminophen (Tylenol) overdose, has mandated new labeling on acetaminophen packaging. It is also considering (but has not enacted) reducing the maximum daily dose from 4 g (possibly to 3,250 mg), banning acetaminophen-narcotic combination products, and changing the current maximum single dose of 1 g to prescription status, making 650 mg the highest recommended nonprescription dose. We review the epidemiology, toxicology, and management of acetaminophen overdose and steps the FDA and physicians can take to prevent it.
KEY POINTS
- Acetaminophen is the leading cause of acute liver failure in the United States, and nearly half of acetaminophenassociated cases are due to unintentional overdose.
- In many cases of unintentional overdose, patients took more than one acetaminophen-containing product and did not know that both products contained this drug.
- Prescribers need to inform all patients, especially vulnerable ones (eg, those taking enzyme-inducing drugs, those who chronically use alcohol, and those who are malnourished) of the risks associated with acetaminophen.
- Although no consensus has been reached on what is a safe dose in patients with liver disease, 4 g/day is too much: a total daily dose of no more than 2 g is recommended to decrease the risk of toxicity in these patients.
MANAGING ACETAMINOPHENHEN OVERDOSE
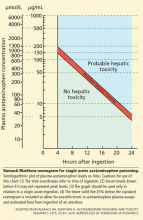
Unintentional overdoses occur over a more prolonged period, and therefore the nomogram is not useful in this situation.
Acetylcysteine is the antidote
Acetylcysteine is the antidote for acetaminophen toxicity and should be given within 8 hours of ingestion for maximal protection against hepatic injury in patients whose serum acetaminophen levels are above the “possible” toxicity line on the nomogram.15 If acetaminophen overdose is suspected but the time elapsed since ingestion cannot be determined, acetylcysteine should be given immediately regardless of the quantity of acetaminophen ingested. 16 In cases of unintentional overdose, it is often given at the discretion of the physician.
Acetylcysteine limits the toxicity of acetaminophen by increasing glutathione stores, binding with NAPQI as a substitute for glutathione, and enhancing sulfate conjugation.6 It may further limit acetaminophen toxicity by nonspecific mechanisms including anti-inflammatory, antioxidant, inotropic, and vasodilating effects.6 In addition, it may prevent further hepatic damage in any patient thought to have acetaminophen-related liver toxicity even beyond the first 12 hours of an overdose.1
Acetylcysteine is available in an oral (Mucomyst) and an intravenous (Acetadote) formulation, which are similar in efficacy. Because many patients find the taste of the oral solution unpleasant and difficult to tolerate, it should be diluted in a 1:3 ratio with cola, orange juice, or other drink to mask its flavor. This mixture should be used within 1 hour of preparation.
Anaphylactoid reactions have occurred in patients receiving intravenous acetylcysteine for acetaminophen overdose soon after the infusion was started, most commonly during the loading dose. The frequency of infusion-related reactions has been reported to be 0.2% to 20.8%.15
The recommended dosage for intravenous acetylcysteine is a loading dose of 150 mg/kg given over 60 minutes, followed by a second dose of 50 mg/kg given over 4 hours, and finally a third dose of 100 mg/kg given over 16 hours.15 For oral acetylcysteine, the loading dose is 140 mg/kg followed by 70 mg/kg every 4 hours for 17 additional doses.17
Other measures
Activated charcoal can be used if the patient presents within 1 to 2 hours after taking acetaminophen. However, the rapid gastrointestinal absorption of acetaminophen makes this treatment ineffective in most cases.9
FINDINGS FROM LARGE REGISTRIES
Findings in adults
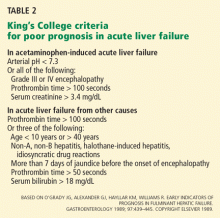
Ostapowicz et al,19 as part of the Acute Liver Failure Study Group, in 2002 prospectively characterized the short-term outcomes of acute liver failure in a large number of patients at 17 tertiary care centers in the United States over approximately 41 months. All centers except one performed liver transplants. Eligible patients had to meet criteria for acute liver failure, including an INR higher than 1.5, evidence of hepatic encephalopathy, and presentation within 26 weeks of illness onset without apparent chronic liver disease.
Of the 308 cases of acute liver failure, 120 (39%) were from acetaminophen overdose, making this drug the most common cause of acute liver failure. Forty-four (37%) of the patients with acetaminophen-related acute liver failure were trying to commit suicide, 57% of cases were accidental, and the remaining reasons for acetaminophen overdose were unknown. The median amount ingested was 13.2 g/day (range 2.6–75 g), and 99 (83%) of the 120 patients took more than 4 g/day.
The patients with acetaminophen-related acute liver toxicity differed from those with other causes of acute liver failure such as idiosyncratic drug reactions or indeterminate causes. The acetaminophen group had a shorter duration of disease and higher serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and serum creatinine than those with acute liver failure from other causes. They also had lower bilirubin levels and a lower arterial pH.
Rates of liver transplantation were 6% in the acetaminophen group, 53% in the group with other drug-induced liver toxicity, 51% in the indeterminate group, and 36% in the remaining groups. Of the acetaminophen group, 47% met the criteria for transplantation, but only 57% of those eligible were listed for transplantation. Those excluded from the transplant list had medical contraindications or were excluded for psychosocial reasons. The short-term transplant-free survival rate was 68% in the acetaminophen group.
Overall, 11% of the patients died, including 28% of the acetaminophen group. The authors concluded that most cases of liver injury in the United States are due to medications and may be preventable.
Larson et al,20 as part of the Acute Liver Failure Study Group, examined the incidence, risk factors, and outcomes of acetaminopheninduced acute liver failure at 22 tertiary care centers in the United States over a 6-year period. Of the 662 patients in the study, 302 had acetaminophen-related toxicity and 275 were included in the final analysis; some of them had been included in the study by Ostapowicz et al.19 During the study period, the number of cases of acute liver toxicity related to acetaminophen increased from 28% to 51%.
Of the patients enrolled in the study, 56% met the criteria for potentially toxic acetaminophen ingestion. Of those who met the criteria, 77% had detectable acetaminophen levels in their serum, and 91% had ALT levels higher than 1,000 IU/L. The time between ingestion and symptom onset ranged from 1 to 32 days, and the median dose was 24 g (range 1.2–180 g).
Of the overdose cases, 48% were unintentional, 44% were intentional, and the remaining 8% had no definable reason. Of the patients in the unintentional-overdose group, 38% were using more than one acetaminophen-containing product and 63% were taking combination products containing narcotics. Overeall, 44% of patients reported using narcotic-acetaminophen combination products. The unintentional-overdose group had lower serum acetaminophen levels than the intentional-overdose group, but they were more likely to present with severe hepatic encephalopathy.
The number of patients with an unintentional overdose was worrisome. Furthermore, one-third of the patients who were receiving an acetaminophen-narcotic combination product were taking an additional acetaminophencontaining product. The authors concluded that unintentional overdose is the leading cause of acetaminophen-related hepatotoxicity, and efforts to limit the over-the-counter package size and to restrict prescriptions of acetaminophen-narcotic combinations may be necessary to decrease the incidence of this preventable cause of acute liver failure.