The polyvagal theory: New insights into adaptive reactions of the autonomic nervous system
ABSTRACT
The polyvagal theory describes an autonomic nervous system that is influenced by the central nervous system, sensitive to afferent influences, characterized by an adaptive reactivity dependent on the phylogeny of the neural circuits, and interactive with source nuclei in the brainstem regulating the striated muscles of the face and head. The theory is dependent on accumulated knowledge describing the phylogenetic transitions in the vertebrate autonomic nervous system. Its specific focus is on the phylogenetic shift between reptiles and mammals that resulted in specific changes to the vagal pathways regulating the heart. As the source nuclei of the primary vagal efferent pathways regulating the heart shifted from the dorsal motor nucleus of the vagus in reptiles to the nucleus ambiguus in mammals, a face–heart connection evolved with emergent properties of a social engagement system that would enable social interactions to regulate visceral state.
CONSISTENCY WITH JACKSONIAN DISSOLUTION
The three circuits are organized and respond to challenges in a phylogenetically determined hierarchy consistent with the Jacksonian principle of dissolution. Jackson proposed that in the brain, higher (ie, phylogenetically newer) neural circuits inhibit lower (ie, phylogenetically older) neural circuits and “when the higher are suddenly rendered functionless, the lower rise in activity.” 18 Although Jackson proposed dissolution to explain changes in brain function due to damage and illness, the polyvagal theory proposes a similar phylogenetically ordered hierarchical model to describe the sequence of autonomic response strategies to challenges.
The human nervous system, similar to that of other mammals, evolved not solely to survive in safe environments but also to promote survival in dangerous and life-threatening contexts. To accomplish this adaptive flexibility, the human nervous system retained two more primitive neural circuits to regulate defensive strategies (ie, fight–flight and death-feigning behaviors). It is important to note that social behavior, social communication, and visceral homeostasis are incompatible with the neurophysiological states and behaviors promoted by the two neural circuits that support defense strategies. Thus, via evolution, the human nervous system retains three neural circuits, which are in a phylogenetically organized hierarchy. In this hierarchy of adaptive responses, the newest circuit is used first; if that circuit fails to provide safety, the older circuits are recruited sequentially.
Investigation of the phylogeny of regulation of the vertebrate heart11,12,19,20 has led to extraction of four principles that provide a basis for testing of hypotheses relating specific neural mechanisms to social engagement, fight–flight, and death-feigning behaviors:
- There is a phylogenetic shift in the regulation of the heart from endocrine communication to unmyelinated nerves and finally to myelinated nerves.
- There is a development of opposing neural mechanisms of excitation and inhibition to provide rapid regulation of graded metabolic output.
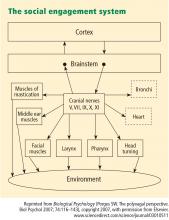
- A face–heart connection evolved as source nuclei of vagal pathways shifted ventrally from the older dorsal motor nucleus to the nucleus ambiguus. This resulted in an anatomical and neurophysiological linkage between neural regulation of the heart via the myelinated vagus and the special visceral efferent pathways that regulate the striated muscles of the face and head, forming an integrated social engagement system (Figure 1; for more details, see Porges7,15).
- With increased cortical development, the cortex exhibits greater control over the brainstem via direct (eg, corticobulbar) and indirect (eg, corticoreticular) neural pathways originating in motor cortex and terminating in the source nuclei of the myelinated motor nerves emerging from the brainstem (eg, specific neural pathways embedded within cranial nerves V, VII, IX, X, and XI), controlling visceromotor structures (ie, heart, bronchi) as well as somatomotor structures (muscles of the face and head).
NEUROCEPTION: CONTEXTUAL CUEING OF ADAPTIVE, MALADAPTIVE PHYSIOLOGICAL STATES
To effectively switch from defensive to social engagement strategies, the mammalian nervous system needs to perform two important adaptive tasks: (1) assess risk, and (2) if the environment is perceived as safe, inhibit the more primitive limbic structures that control fight, flight, or freeze behaviors.
Any stimulus that has the potential for increasing an organism’s experience of safety has the potential of recruiting the evolutionarily more advanced neural circuits that support the prosocial behaviors of the social engagement system.
The nervous system, through the processing of sensory information from the environment and from the viscera, continuously evaluates risk. Since the neural evaluation of risk does not require conscious awareness and may involve subcortical limbic structures,21 the term neuroception22 was introduced to emphasize a neural process, distinct from perception, that is capable of distinguishing environmental (and visceral) features that are safe, dangerous, or life-threatening. In safe environments, autonomic state is adaptively regulated to dampen sympathetic activation and to protect the oxygen-dependent central nervous system, especially the cortex, from the metabolically conservative reactions of the dorsal vagal complex. However, how does the nervous system know when the environment is safe, dangerous, or life-threatening, and which neural mechanisms evaluate this risk?
Environmental components of neuroception
Neuroception represents a neural process that enables humans and other mammals to engage in social behaviors by distinguishing safe from dangerous contexts. Neuroception is proposed as a plausible mechanism mediating both the expression and the disruption of positive social behavior, emotion regulation, and visceral homeostasis.7,22 Neuroception might be triggered by feature detectors involving areas of temporal cortex that communicate with the central nucleus of the amygdala and the periaqueductal gray, since limbic reactivity is modulated by temporal cortex responses to the intention of voices, faces, and hand movements. Thus, the neuroception of familiar individuals and individuals with appropriately prosodic voices and warm, expressive faces translates into a social interaction promoting a sense of safety.
In most individuals (ie, those without a psychiatric disorder or neuropathology), the nervous system evaluates risk and matches neurophysiological state with the actual risk of the environment. When the environment is appraised as being safe, the defensive limbic structures are inhibited, enabling social engagement and calm visceral states to emerge. In contrast, some individuals experience a mismatch and the nervous system appraises the environment as being dangerous even when it is safe. This mismatch results in physiological states that support fight, flight, or freeze behaviors, but not social engagement behaviors. According to the theory, social communication can be expressed efficiently through the social engagement system only when these defensive circuits are inhibited.
Other contributors to neuroception
The features of risk in the environment do not solely drive neuroception. Afferent feedback from the viscera provides a major mediator of the accessibility of prosocial circuits associated with social engagement behaviors. For example, the polyvagal theory predicts that states of mobilization would compromise our ability to detect positive social cues. Functionally, visceral states color our perception of objects and others. Thus, the same features of one person engaging another may result in a range of outcomes, depending on the physiological state of the target individual. If the person being engaged is in a state in which the social engagement system is easily accessible, the reciprocal prosocial interactions are likely to occur. However, if the individual is in a state of mobilization, the same engaging response might be responded to with the asocial features of withdrawal or aggression. In such a state, it might be very difficult to dampen the mobilization circuit and enable the social engagement system to come back on line.
The insula may be involved in the mediation of neuroception, since it has been proposed as a brain structure involved in conveying the diffuse feedback from the viscera into cognitive awareness. Functional imaging experiments have demonstrated that the insula plays an important role in the experience of pain and the experience of several emotions, including anger, fear, disgust, happiness, and sadness. Critchley proposes that internal body states are represented in the insula and contribute to states of subjective feeling, and he has demonstrated that activity in the insula correlates with interoceptive accuracy.23