Pinacidil induces vascular dilation and hyperemia in vivo and does not impact biophysical properties of neurons and astrocytes in vitro
ABSTRACT
Vascular and neural systems are highly interdependent, as evidenced by the wealth of intrinsic modulators shared by the two systems. We tested the hypothesis that pinacidil, a selective agonist for the SUR2B receptor found on smooth muscles, could serve as an independent means of inducing vasodilation and increased local blood volume to emulate functional hyperemia. Application of pinacidil induced vasodilation and increased blood volume in the in vivo neocortex in anesthetized rats and awake mice. Direct application of this agent to the in vitro neocortical slice had no direct impact on biophysical properties of neurons or astrocytes assessed with whole-cell recording. These findings suggest that pinacidil provides an effective and selective means for inducing hyperemia in vivo, and may provide a useful tool in directly testing the impact of hemodynamics on neural activity, as recently predicted by the hemo-neural hypothesis.
Interactions between the brain and blood are essential to health. Metabolic supply of the brain is provided through the vasculature, and disruptions of this relationship, in extreme cases such as stroke, is a key characteristic of neurologic disease. Neuro-hemodynamic coupling is also demonstrated in healthy individuals on faster time scales in functional hyperemia, the local increase in blood flow and volume that accompanies neural activity.1,2
We have recently proposed a further level of interdependence between the two systems —ie, the hemoneural hypothesis—which predicts that hemodynamic events such as functional hyperemia will modulate neural activity.3 An impact of hemodynamics on neurons could occur through a number of mechanisms, including the activation of mechanoreceptors on astrocytes or neurons, a thermal impact of increased blood flow on ion channels and vesicle release, and the local increase and diffusion of blood-borne factors such as nitric oxide.4,5 Astrocytes are predicted to play a key role in hemo-neural modulation, as they are tightly coupled to the vascular system and participate in a number of neural functions.6,7 Through these mechanisms and others, hemodynamics could shift the “state” of the local neural circuit, thereby impacting information processing. This regulation of neural dynamics could also provide a homeostatic mechanism for promoting healthy brain function (eg, prevention of kindling).
To study the impact of hyperemia on neural and astrocytic activity in vivo, it is essential to independently control blood flow in the brain with means that do not directly impact neurons or astrocytes. Pinacidil is a sulfonylurea receptor agonist that opens the SUR2B potassium-sensitive ATP channel.8 In the telencephalon, SUR1 receptors are localized to neurons and glia.9,10 In contrast, SUR2 receptors are localized to vasculature, with SUR2A in cardiac and skeletal muscle, and SUR2B in vascular smooth muscle, with primary expression in smaller arteries, arterioles, and capillaries.11 By opening the SUR2B channel, pinacidil hyperpolarizes and relaxes smooth muscle, causing vasodilation. Pinacidil is a potent and selective SUR2B agonist, with a dissociation constant of 135 nM and a half maximal effective concentation (EC50) value of 680 nM.12 This agonist is approximately 5 times more specific for SUR2B than for SUR2A and shows approximately 5 orders of magnitude lower affinity for SUR1 (in the mM range).12–15 Previous studies have demonstrated the efficacy of this agent as a vasodilator.16–18
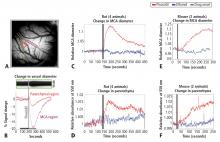
In the present study, we systematically examined the utility of pinacidil for the selective induction of hyperemia. First, we quantified the vasodilation induced by pinacidil in vivo, and examined local increases in blood volume in the parenchyma. These studies were conducted in anesthetized rats and awake mice. Second, we used in vitro slice recordings to examine whether direct application of relatively high concentrations of pinacidil would have any impact on the physiology of neurons and astrocytes. We found that (1) in vivo, pinacidil induces a level of vasodilation and increased local blood volume consistent with natural functional hyperemia across a variety of preparations, and (2) in vitro, pinacidil has no detectable impact on intrinsic biophysical measures in neurons and astrocytes.
METHODS
Animal preparation in vivo
To probe the impact of pinacidil on arterial diameter and parenchymal blood volume in vivo, we measured the effects of topical application to the primary somatosensory cortex (SI) of rats and mice. Sprague-Dawley rats (250–500 g) and C57BL/6 mice (~25 g) were anaesthetized with pentobarbital (50 mg/kg intraperitoneally initial dose, followed by 5-mg supplements as needed for maintenance). Animals were maintained at approximately 37°C by a heating blanket. Craniotomy (diameter of ~2 mm in rats, ~1 mm in mice) and durotomy were performed over SI, and the cortex was protected with Kwik-Cast silicone elastomer sealant (WPI, Sarasota, FL) while an imaging chamber was attached with dental cement. Kwik-Cast was removed, and the chamber filled with 0.9% saline and sealed with a round cover glass (avoiding bubbles) secured with cyanoacrylate.
Controlling visualization during drug delivery in vivo
To minimize brain motion and flow artifacts during visualization of hemodynamics in the rat preparation, we constructed a customized pressurized chamber with inflow and outflow for constant perfusion. The volume of the chamber was approximately 0.3 mL, and the flow through the system averaged about 2 mL/min. The chamber consisted of a plastic ring 1 cm in diameter and 3 mm high with a flat-top profile and a base shaped to the angle of the lateral skull edge over SI. In the wall of this chamber, three large holes were drilled and patched with pieces cut from rubber NMR septa (VWR International, West Chester, PA) to create resealable ports for drug application and bubble removal. Three additional permanent holes were drilled in the chamber walls, through which blunted 1-cm lengths of 18-gauge stainless steel needles were wedged and affixed with Super glue: one for artificial cerebrospinal fluid (ACSF) inflow, one for combined outflow, and the third for pressure regulation. The overall pressure of the chamber was regulated by a small vertical tube whose height (and thus fluid level) could be adjusted on a manipulator stand, and whose other end was open to the atmosphere. Inflow and outflow were controlled via regulators on a gravity feed system. In the mouse preparation, the need to control visualization was addressed by maintaining a constant rate of wicking in a smaller-profile open chamber, and a microfluidic switch with 12 μL of dead space was added to minimize propulsive impact and delay due to switching between solutions. Drug and ethanol solutions were delivered to rat and mouse chambers after being heated to physiological temperature (37°C).
Optical measurement of hemodynamics in vivo
We used a charge-coupled device camera (the Roper 512B, Princeton Instruments, Trenton, NJ) to image the cortical surface at a frame rate of approximately 4 Hz, with illumination from a voltage-regulated xenon arc lamp. A green band-pass filter (550 nm) was used to maximize imaging near the isosbestic point of hemoglobin, providing optimal vessel contrast and a surrogate measure for blood volume change in the parenchyma. Lenses (50 and 125 mm) were arranged in series to form a macroscope.