Neuromodulation of cardiac pain and cerebral vasculature: Neural mechanisms
ABSTRACT
Research using animal models has helped elucidate the neural mechanisms of angina pectoris, sensitization of cardiac nociceptive stimuli, and neuromodulation of cardiac pain and cardiovascular function. Findings over the last 2 decades include evidence of convergence of visceral-somatic input to spinothalamic cells and a major role for the vagus nerve in spinal cord processing. Stress-related glucocorticoids may manipulate amygdala function, inducing hypersensitivity to nociceptive input from the heart via central sensitization of upper thoracic spinal neuronal activity. Spinal cord stimulation may have therapeutic effects, although the underlying mechanism is unclear.
The cardinal symptoms of angina pectoris—chest pain and pain that may radiate to either arm or the neck and jaw—are well recognized. The visceral characteristics of anginal pain are also familiar; for example, referral to somatic structures, pain that is diffuse and poorly localized, skin and deep tissue tenderness, enhanced autonomic reflexes such as sweating and vasomotor symptoms, and muscular rigidity.
The neurologic mechanisms that explain the manifestations of angina pectoris are less well clarified, and are targets of active research. Our research into the neuromodulation of cardiovascular function over the last 2 decades has produced results that may have clinical implications and others that have raised new questions. This article summarizes some of our key findings from studies of neural mechanisms of angina pectoris, central sensitization of cardiac nociceptive stimuli, and the neuromodulation of cardiac pain, with a focus on processing in the spinal cord.
NEURAL MECHANISMS OF ANGINA PECTORIS
Cells of the spinothalamic tract form a sensory pathway that transmits afferent information to the thalamus.1 One of our research objectives was to examine how these cells process information when the heart is exposed to noxious stimuli.
Thoracic spinal processing
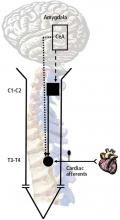
The animal model for our early studies was an anesthetized primate. The afferent nerves were activated in one of two ways: either the coronary artery was occluded or bradykinin and algesic chemicals were injected into the pericardial sac or left atrial appendage. Recorded activity was then made from the spinothalamic tract cells in the T1–T5 and C5–C6 segments.1 We found convergence of visceral and somatic input, generally to the chest and upper arm. The finding was consistent with the observation that pain from angina commonly occurs in proximal somatic fields. No visceral input was evident in cells in C7–C8, where the somatic effects are primarily distal—to the hand, for example.
Upper cervical processing
It is known that some patients experience angina pectoris as neck and jaw pain. The dental literature has shown that what is initially considered to be a toothache occasionally turns out to be angina and coronary artery disease.2 Clinical literature from the late 1940s observed that despite the use of sympathectomy to relieve angina pectoris, neck and jaw pain continued or developed.3,4 This pain was attributed to transmission of nociceptive information in vagal afferent fibers, commonly thought to transmit innocuous cardiac sensory information.
When we recorded activity from spinothalamic tract cells in the C1–C2 region to observe the effect of cardiac nociceptive stimulation, we demonstrated a major role for the vagus nerve.1 Injection of saline into the heart had no effect in the C1–C2 region, but injection of algesic chemicals into the pericardial sac caused significant activity that disappeared after transection of the vagus nerve. This finding suggested that vagal afferent fibers ascend into the nucleus tractus solitarius of the medulla and either directly or indirectly modulate the C1–C2 neurons, which also receive converging somatic information from the neck and jaw region.5
CENTRAL SENSITIZATION OF CARDIAC NOCICEPTIVE STIMULI
Clinical studies suggest that anxiety and depression are prevalent in patients suffering from chest pain with and without underlying cardiac disease.6 Anxiety and/or stress increases circulating levels of corticosteroids, which can act on the glucocorticoid receptors in the amygdala, particularly in the central area.7 The amygdala plays a pivotal role in transforming chronic stressful stimuli into behavioral, visceral, and autonomic responses.8
Previous studies have shown that corticosteroids upregulate expressions of corticotropin-releasing factor in the central nucleus of the amygdala and increase indices of anxiety.7,9 They are also associated with hypersensitivity in visceromotor responses to colorectal distention10 and sensitize lumbosacral spinal neurons to colorectal and urinary bladder distention.11,12 We therefore hypothesized that glucocorticoids manipulate amygdala function, inducing hypersensitivity to nociceptive input from the heart through the modulation of upper thoracic spinal neuronal activity.
To examine the impact of stress on the nervous system when the heart is exposed to noxious stimuli, we assessed the effect of chronic activation of the amygdala on the T3–T4 spinal neurons and on C1–C2 propriospinal neurons. Fisher 344 rats were selected for this study because of their relatively low level of anxiety-related behavior.9 Micropellets of crystalline corticosterone or cholesterol (30 μg, used as a control) were implanted in the central nucleus of the amygdala. After 7 days, the corticosterone-implanted, but not the cholesterol-implanted, animals displayed high-anxiety behavior, as determined with an elevated plus maze.7
The responses of T3–T4 spinal neurons to intrapericardial injections of the algesic chemical bradykinin were compared in the corticosterone- and cholesterol-implanted rats. Compared with cholesterol-implanted animals, the duration of activity in response to the noxious cardiac stimulus was significantly longer in the corticosterone-implanted rats; in addition, activity shifted from the short-lasting (the response lasts only as long as the stimulus is applied) to long-lasting excitatory (the response lasts well beyond the period the stimulus is applied) neurons. Long-lasting excitatory neuronal activity is associated with intense pain and hypersensitivity, while short-lasting neurons are associated with a more acute response. The number of neurons with large field sizes in the corticosterone-implanted animals also increased, which is another indication of sensitization.