Pioneer Award Address: Ignorance isn’t biased: Comments on receiving the Pioneer Award
ABSTRACT
Researchers ordinarily work by deriving testable hypotheses from theories using a deductive process. Hypothesis testing is inherently biased, however, because of the practical requirements of finding and publishing positive results. In contrast, ignorance isn’t biased. The combination of relevant new technology, sufficient mastery of the topic to know what is not yet known, and access to patients with rare but informative disorders sets the stage for discoveries about disease mechanisms based on induction from observations. Patient-oriented research is a strength of heart-brain medicine. Patients are a unique scientific resource because they tell us the truth. We experience the joy and thrill of a “sparkle of insight” when we realize what they teach.
Beyond a brain disease: Seeing PD as a heart-brain disorder
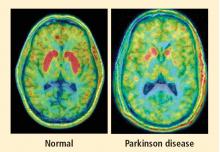
More than 50 neuroimaging studies since our original report have agreed remarkably consistently on the association between PD and loss of sympathetic nerves in the heart; moreover, postmortem pathology studies have amply confirmed that a profound loss of cardiac sympathetic nerves is characteristic of PD.16 I have yet to come across a single patient with PD and orthostatic hypotension who has not had cardiac sympathetic denervation, and virtually all patients with PD who do not have orthostatic hypotension seem to have at least partial loss of cardiac sympathetic nerves.
Considering that the source of those nerves is the ganglia, which lie outside the central nervous system, PD must be more than a brain disease and more than a movement disorder. It must also be a disease of the sympathetic nerves in the heart, a form of a dysautonomia, and a heart-brain disorder.
The role of catecholamines: Another discovery born of unbiased ignorance
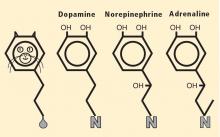
Almost a half century ago, Hornykiewicz and colleagues made the pivotal discovery that PD features loss of dopamine in the nigrostriatal system in the brain.18 Given the cardiac sympathetic denervation, PD might be a disease of catecholamine systems both inside and outside the central nervous system—dopamine in the nigrostriatal system, and norepinephrine in the sympathetic nerves of the heart.
Then what of the third catecholamine, adrenaline, in PD? Plasma levels of adrenaline and of its metabolite, metanephrine, are normal in PD, even in patients who have PD and orthostatic hypotension, which involves loss of norepinephrine-producing nerves not only in the heart but in other organs.19 What is different about the adrenaline-producing cells in the medulla (from the Latin for “marrow”) of the adrenal glands atop each kidney? Why aren’t these catecholamine-producing cells also lost in PD?
I have some ideas in mind but won’t go into them here. The point is that the discovery of normal adrenaline-producing cells in PD, despite loss of cells producing the other catecholamines, was not based on my testing a hypothesis. It was a discovery born of ignorance, and because ignorance isn’t biased, that discovery points to the truth. Whatever the eventual explanation for the specific pattern of catecholamine cell loss in PD, it cannot refute the discovery itself.
HOW DISCOVERIES ARISE: AN APPLIED EXERCISE FOR READERS
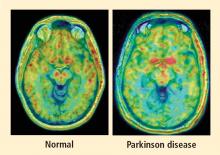
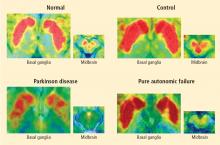
PAF is a rare disease, and I have only studied several cases with high-resolution PET scanning of the brain, but so far they have all had this unexpected, unpredicted finding of loss of dopaminergic neurons in the substantia nigra.20
What does this pattern mean? If PAF patients have just as much loss of nigral neurons as PD patients do, and if PAF patients do not have parkinsonism, then the movement disorder in PD cannot result from loss of the dopamine neurons in the substantia nigra per se. Instead, the movement disorder in PD seems to come from loss of the dopaminergic terminals in the striatum.
How can PAF patients have normal dopamine terminals in the putamen when the number of dopaminergic cell bodies is severely reduced? Somehow, PAF patients must be able to sprout new terminals, even as they lose the cell bodies. Maybe if we knew how PAF patients do this, we would have a way to treat or even prevent PD.
How do PAF patients maintain normal dopamine terminals as the cell bodies die off? No one knows. Until now, no one thought of asking such a question. No one hypothesized that this discovery would be made, but it was. And because ignorance isn’t biased, we have put our finger on the truth. By keeping in mind what isn’t known, we could see what wasn’t there. Now we can begin to think of what to look for next.