Influenza in long-term care facilities: Preventable, detectable, treatable
ABSTRACTInfluenza in long-term care facilities is an ever more challenging problem. Vaccination of residents and health care workers is the most important preventive measure. Although vaccine efficacy has been questioned, the preponderance of data favors vaccination. Antiviral resistance complicates postexposure chemoprophylaxis and treatment. Factors that limit the choice of antiviral agents in this patient population include limited vaccine supplies and impaired dexterity and confusion in long-term care residents.
KEY POINTS
- When health care workers in long-term care facilities are vaccinated against influenza, significantly fewer residents die or develop influenza-like illness, particularly when residents are also vaccinated.
- Easily accessible dispensers for alcohol-based antiseptic foam or gel can significantly improve hand hygiene rates in health care workers.
- If a patient in a long-term care facility is visibly coughing and cannot cover his or her mouth, health care workers should wear a mask when within 3 feet of the patient.
- All isolates of pandemic influenza A/H1N1 (previously called swine-origin influenza virus) are susceptible to zanamivir (Relenza) and oseltamivir (Tamiflu), but are resistant to amantadine (Symmetrel) and rimantadine (Flumadine).
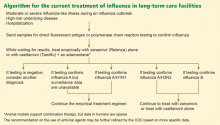
TREATMENT OF INFLUENZA IN LONG-TERM CARE FACILITIES
A mild illness in someone without a high-risk underlying disease can be managed with symptomatic measures alone, and testing for influenza is at the discretion of the caregiver. Patients who develop a moderate or severe influenza-like illness during an influenza epidemic and who have a high-risk underlying disease or who require hospitalization should be tested for influenza by a sensitive test such as direct fluorescent antigen testing or PCR and should be started on empiric anti-influenza therapy.41
Most elderly residents of long-term care facilities do have comorbid conditions and are thus at high risk of complications of influenza. During influenza outbreaks, clinicians caring for these patients—and for similar patients in outpatient settings, emergency departments, and acute-care hospitals—should consider testing for influenza, even if the patient has only a mild influenza-like illness.
Of note, no randomized treatment study has been done specifically in residents of long-term care facilities.52 Pooled analysis of data from 321 patients at high risk (76 were age 65 or older) in zanamivir treatment studies showed that those who received the drug were sick for 2.5 fewer days than those who received placebo.53 In addition, zanamivir recipients returned to normal activities 3 days sooner, had an 11% reduction in the median total symptom score over 1 to 5 days, and had a rate of complications requiring antibiotics 43% less than placebo recipients. Rates of adverse events were similar in both groups.
A multicenter, randomized, open-label, controlled trial of oseltamivir in the treatment of influenza in high-risk Chinese patients with chronic respiratory diseases (chronic bronchitis, obstructive emphysema, bronchial asthma, or bronchiectasis) or chronic cardiac disease showed that oseltamivir significantly reduced the duration of influenza symptoms by 36.8%, the severity of symptoms by 43.1%, the duration of fever by 45.2%, the time to return to baseline health status by 5 days, and the need for antibiotics, without increasing the total cost of medical care.54
Oseltamivir-resistant isolates of seasonal influenza A/H1N1 do not cause different or more severe symptoms than do oseltamivir-susceptible isolates, and they remain susceptible to zanamivir and the adamantanes. There is no widely available test to differentiate influenza A/H1N1 from A/H3N2 or to test for drug resistance. Influenza B and A/H3N2 remain susceptible to both of the neuraminidase inhibitors, ie, oseltamivir and zanamivir.
All currently circulating influenza A/H3N2 isolates remain resistant to the adamantanes. Influenza B is intrinsically resistant to the adamantanes, and some data show that oseltamivir is less effective against influenza B than against influenza A.
Zanamivir is not available in many pharmacies, is not recommended in patients with underlying reactive airway disease, and requires dexterity for administration.
Amantadine is more likely to cause confusion in the elderly than rimantadine, and it is more susceptible to treatment-emergent antiviral resistance.
No parenteral anti-influenza drug is available, but zanamivir and the experimental drug peramivir are undergoing study for parenteral use. An inhaled, long-acting neuraminidase inhibitor, CS-8958, is currently under study.55 Other agents currently under development include T-705, a polymerase inhibitor, and DAS181, an attachment inhibitor.
Complementary and alternative therapies for influenza are not established. A recent review of 14 randomized, controlled trials56 revealed that the evidence is weak and limited by small sample sizes, poor methodologic quality, or clinically irrelevant effect sizes.