The resurgence of swine-origin influenza A (H1N1)
ABSTRACTUnexpectedly, swine-origin influenza A (H1N1) virus (S-OIV, informally known as swine flu) appeared in North America at the very end of the 2008–2009 influenza season and began to spread internationally. As the world mobilizes for a potential pandemic, this article summarizes the developments in diagnosis, treatment, and prevention.
KEY POINTS
- What happens in the annual influenza season in the Southern Hemisphere will indicate the prospects of S-OIV progressing to a pandemic.
- Oseltamivir (Tamiflu) and zanamivir (Relenza) are active against S-OIV and are recommended for hospitalized patients or people at higher risk of influenza-related complications.
- Otherwise-healthy patients who present with an uncomplicated febrile illness due to S-OIV do not require antiviral treatment.
- Hand-washing is the most important preventive measure.
- Vaccine development may take 4 to 6 months. The most difficult question about vaccine development for S-OIV is whether to prepare it as a separate product or incorporate it in the seasonal influenza vaccine.
VACCINE DEVELOPMENT
The most difficult question about vaccine development for S-OIV at this time is whether to prepare it as a separate product or try to incorporate it in the seasonal influenza vaccine.
The problem is that the seasonal influenza vaccine for the Southern Hemisphere has already been made and distributed, and vaccination programs are already well under way. Although flu season in the Northern Hemisphere is not expected before September or October 2009, vaccine production and distribution take several months, leaving little time to observe which direction the S-OIV epidemic will take before making this decision.
Vaccine distribution also raises difficult questions, since a limited amount will be available initially and rationing to the most vulnerable people will be necessary. While health care workers are more likely to be exposed to people infected with S-OIV compared with the general population, mandating their immunization may pose other moral dilemmas.17
The current global capacity for production of seasonal influenza vaccine is approximately 400 million doses.18 Since the process of vaccine production takes at least 4 to 6 months, measures have been proposed to speed up the production of pandemic vaccine or immunogenicity; these include recombinant technology, reverse genetics, and the use of adjuvants. In April 2007, the FDA approved the first H5 subviron vaccine for people ages 19 to 64.
This topic brings back memories of the 1976 swine influenza immunization program, in which the rate of Guillain-Barré syndrome was 5 to 10 times the background rate, resulting in a halt in vaccine production.
Why this syndrome occurred is not known, but it is suspected to be due to cross-reacting antibodies against peripheral-nerve antigen that developed after the vaccine was given. Data since then have shown no association between vaccination and Guillain-Barré syndrome. 19 On the other hand, influenza viruses were found to trigger Guillain-Barré syndrome only infrequently, except during major outbreaks, in which they may play a significant role.20
TREATMENT
Antiviral drugs
Tests of current S-OIV isolates showed them to be susceptible to the neuraminidase inhibitors, ie, oseltamivir and zanamivir, but resistant to the adamantanes, ie, amantadine (Symmetrel) and rimantadine (Flumadine).21 All isolates contained the S31N mutation in the M2 protein, which confers resistance against the adamantanes and which has been detected in most influenza A (H3N2) isolates in the United States since 2006. Fortunately, the H274Y mutation in N1—which confers resistance to oseltamivir but not to zanamivir and which has been detected in almost all seasonal influenza A (H1N1) isolates since the early weeks of the current influenza season— has not been detected in any of the current S-OIV isolates.
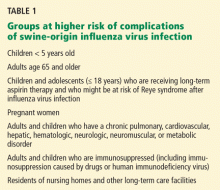
Patients who are otherwise healthy who present with an uncomplicated febrile illness due to S-OIV do not require antiviral treatment. Either oseltamivir or zanamivir is recommended for treatment of patients hospitalized for management of confirmed, probable, or suspected infection with S-OIV, or for those at high risk of influenza-related complications, defined similarly to seasonal influenza (Table 1).
The duration of shedding of S-OIV is unknown, but starting an antiviral agent early in the course of illness is expected to reduce contagiousness. Extrapolating from data in seasonal influenza, infected persons are assumed to be shedding virus from 1 day prior to illness onset until resolution of symptoms, usually 7 days, and up to 10 days in younger children.
Oseltamivir accounts for the lion’s share of the stockpile of antiviral drugs against pandemic influenza. However, with mass utilization, antiviral resistance to a single agent may develop. A mathematical model showed that adding a smaller stockpile of a second agent, such as zanamivir, to be used either in combination with or sequential to oseltamivir, can effectively prevent or at least delay the development of resistance.22
Other potential measures for management
Since secondary bacterial pneumonia is expected to play a significant role in influenza-related death during the next pandemic, stockpiling antibacterial agents may also be prudent.8 The death rate in methicillin-resistant Staphylococcus aureus pneumonia secondary to seasonal influenza is 50%, further complicating the choice of stockpiling for antibacterial agents.
A meta-analysis of 11 studies involving 1,703 patients during the 1918 pandemic showed that those who received influenza-convalescent human blood products were less likely to die than those who did not.23 Anti-influenza drugs and advanced techniques to care for critically ill patients were not available at that time, so extrapolating these data to the current era may not be appropriate.
The cost of vaccine and antiviral drugs is an expected limitation to mass implementation during a pandemic, particularly in developing countries. Certain inexpensive generic drugs that have been shown to have some activity against influenza, such statins, fibrates, and chloroquine, deserve further attention.24
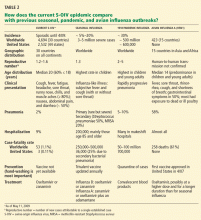
PUTING THE CURRENT EPIDEMIC IN PERSPECTIVE
In summary, the world is now better prepared, vaccine is in development, and antiviral treatment is available. For more information, readers are directed to go to www.cdc.gov/h1n1flu/ or www.who.int/csr/don/2009_05_11/en/index.html.