Acute pancreatitis: Problems in adherence to guidelines
ABSTRACTAlthough evidence-based guidelines on managing acute pancreatitis are available, many physicians are not following them. The authors identify and discuss several problems in adherence to guidelines on testing, imaging, and treatment.
KEY POINTS
- Serum amylase and lipase levels are often needlessly measured every day.
- Often, severity assessments are not performed regularly or acted on.
- Often, not enough fluid is replaced, or fluid status is not adequately monitored.
- In many severe cases, enteral or parenteral feeding is not started soon enough.
- Computed tomography is not done in many patients with severe acute pancreatitis, or it is performed too soon.
- In many cases of suspected infected necrosis, fine-needle aspiration is not done.
- Broad-spectrum antibiotics are often used inappropriately in patients with mild acute pancreatitis and in patients with sterile necrotizing pancreatitis who are clinically stable and have no signs of sepsis.
Recommendation: Early, aggressive fluid replacement
Experts have suggested initially infusing 500 to 1,000 mL of fluid per hour in those who are volume-depleted, initially infusing 250 to 350 mL per hour in those who are not volumedepleted, and adjusting the fluid rate every 1 to 4 hours on the basis of clinical variables.19 The sufficiency of fluid replacement should be carefully monitored by vital signs, urine output, and serum hematocrit.
On the other hand, overly aggressive fluid resuscitation can be detrimental in patients at risk of volume overload or pulmonary edema. Fluid replacement should be tempered in elderly patients and those with cardiac or renal comorbidities, and may require monitoring of central venous pressure.
The ACG and AGA guidelines both recognize the need for early aggressive volume replacement in acute pancreatitis (level of evidence III), but they do not specify the exact amounts and rates. Young and healthy patients should receive a rapid bolus of isotonic saline or Ringer’s lactate solution followed by an infusion at a high initial maintenance rate.
Few studies have been done to assess physicians’ compliance with recommendations for aggressive volume replacement. In an Italian multicenter study, patients with mild or severe acute pancreatitis received an average of only 2.5 L of fluid per day (about 100 mL/hour).20 Gardner et al21 recently summarized the available evidence for fluid support in acute pancreatitis.
NUTRITIONAL SUPPORT
Problem: In many severe cases, enteral or parenteral feeding is not started soon enough.
Nutritional support entails enteral or parenteral feeding when an oral diet is contraindicated. Enteral feeding is usually via a nasojejunal tube, which may need to be placed under endoscopic or radiographic guidance. Neither parenteral nor nasojejunal feeding stimulates pancreatic secretion, and both are safe in acute pancreatitis.
Severe acute pancreatitis is an intensely catabolic state characterized by increased energy expenditure, protein breakdown, and substrate utilization. Patients may not be able to resume an oral diet for weeks or even months, particularly if local complications develop. Early nutritional support has been shown to improve outcomes in severe acute pancreatitis.22 Therefore, nutritional support should be started as soon as possible in severe acute pancreatitis based on initial clinical and radiographic indicators of severity, optimally within the first 2 or 3 days.
Enteral nutrition is preferred to parenteral nutrition in pancreatitis: it is less expensive and does not pose a risk of catheter-related infection or thrombosis or hepatic complications. Also, there is experimental evidence that enteral nutrition may preserve the gut barrier, decreasing mucosal permeability and bacterial translocation.
A number of small randomized trials compared enteral and parenteral nutrition in acute pancreatitis, but they yielded mixed results. A meta-analysis of six trials showed a lower rate of infectious complications with enteral than with parenteral nutrition. 23 However, no significant difference was found in the rates of death or noninfectious complications.
Recommendation: Enteral feeding, when possible
Nutritional support is unnecessary in most cases of mild acute pancreatitis. Pancreatic inflammation typically resolves within a few days, allowing patients to resume eating. Occasionally, patients in whom pain resolves slowly and who fast for more than 5 to 7 days need nutritional support to prevent proteincalorie malnutrition.
The ACG guidelines1 and most others suggest that, whenever possible, enteral rather than parenteral feeding should be given to those who require nutritional support. The level of evidence is II (“strong evidence from at least one published properly designed randomized controlled trial of appropriate size and in an appropriate clinical setting”).
However, not all physicians recognize the benefit of enteral feeding. In a cohort of German gastroenterologists, only 73% favored enteral over parenteral feeding in acute pancreatitis.7
COMPUTED TOMOGRAPHY
Problem: CT is not done in many patients with severe acute pancreatitis, or it is done too soon during the admission.
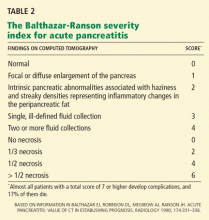
Dual-phase, contrast-enhanced, pancreatic-protocol CT provides a sensitive structural evaluation of the pancreas and is useful to diagnose necrotizing pancreatitis. Pancreatic necrosis is correlated with a severe clinical course, the development of single or multiorgan dysfunction, and death.
Recommendation: CT in severe cases
Not every patient with acute pancreatitis needs to undergo CT. Most mild cases do not require routine CT, since necrosis and other local complications are infrequent in this group.
Also, CT is often ordered too soon during the hospitalization. Indicators of severity on CT are not usually evident until 2 to 3 days after admission.25 CT should be considered about 3 days after the onset of symptoms rather than immediately upon admission.
On the other hand, CT at the time of admission may be warranted to rule out other life-threatening causes of abdominal pain and hyperamylasemia (eg, bowel obstruction, viscus perforation). CT may also be useful in the late phase of acute pancreatitis (weeks after admission) to diagnose or monitor complications (eg, pseudocysts, abscesses, splenic vein thrombosis, splenic artery pseudoaneurysms). Magnetic resonance imaging with gadolinium contrast is a reasonable alternative to CT for detecting pancreatic necrosis and other local complications.
In patients who have severe acute pancreatitis and compromised renal function (serum creatinine > 1.5 mg/dL), CT can be performed without contrast to assess severity based on a limited Balthazar score (ie, without a necrosis score). Studies in rats suggest that iodinated contrast may decrease pancreatic microcirculation and worsen or precipitate necrosis,26 although published human studies do not support this contention.27,28
Guidelines uniformly recommend CT for patients with severe acute pancreatitis (the ACG guideline gives it a level of evidence of III), but this recommendation is not always followed. A study from Australia showed that CT was done in only 27% to 67% of patients with severe acute pancreatitis.5 In a British study, only 8 of 46 patients with clinically predicted severe pancreatitis underwent CT within the first 10 days of admission.4