The role of hyperuricemia and gout in kidney and cardiovascular disease
ABSTRACT
Elevated serum urate levels are recognized as leading to gouty arthritis, tophi formation, and uric acid kidney stones. While serum urate elevations have long been associated with renal disease, they are not usually considered to have a causal role in kidney dysfunction. However, recent epidemiologic studies have identified serum urate elevations as an independent risk factor for chronic kidney disease. Hyperuricemia has also been found to be an independent risk factor for cardiovascular disease and hypertension. An animal model of mild hyperuricemia has shed new light on a potential mechanism of microvascular changes leading to endothelial dysfunction, a precursor to both coronary artery disease and hypertension. Additional animal studies and recent epidemiologic findings have provided further evidence that soluble urate is a risk factor for nonarticular disease.
KEY POINTS
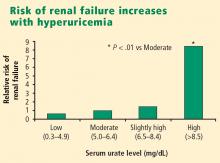
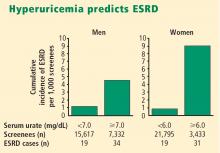
- Hyperuricemia significantly increases the risk of renal failure and end-stage renal disease.
- Larger, more rigorous trials are under way to assess preliminary findings suggesting that urate-lowering therapy might normalize blood pressure in hypertensive adolescent patients.
- Use of urate-lowering therapies to treat hyperuricemia is not currently supported in patients with kidney disease, heart disease, or hypertension in the absence of gout or uric acid kidney stones.
Hyperuricemia is a metabolic problem that has become quite common over the past several decades. The main clinical issues associated with hyperuricemia are gouty arthritis, gouty tophi, and uric acid kidney stones. For decades, these have been the main indications for lowering serum urate levels. Well-established nonarticular associations of hyperuricemia in gout include chronic kidney disease, coronary artery disease, and hypertension.1 Recent animal studies and epidemiologic studies have shed new light on the relationship between urate and comorbid disease processes. This article describes our evolving understanding of the association of hyperuricemia and gout with kidney disease, hypertension, and cardiovascular disease, and also reviews the kidney’s role in regulation of urate levels in the body.
SOURCES, DISTRIBUTION, AND ELIMINATION OF URATE
Uric acid is the end product of purine metabolism in humans. Sources of purine are either endogenous, from de novo synthesis and nucleic acid breakdown (approximately 600 mg/day), or exogenous, from dietary purine intake (approximately 100 mg/day).2 In the steady state, this daily production and ingestion of approximately 700 mg of uric acid is balanced by daily elimination of an equal amount of uric acid from the body. Approximately 30% of this daily loss is through the gut, with subsequent bacterial intestinal uricolysis. The other 70% (roughly 500 mg daily) needs to be excreted by the kidneys.
In humans, plasma urate is freely filtered at the glomerulus, but the fractional excretion of the filtered uric acid is less than 10%. This demonstrates a dominance of reabsorptive processes in humans, and these processes are handled primarily by the selective urate transport protein known as URAT1 in the proximal convoluted tubules. In normouricemic individuals, there is a balance between the daily production and ingestion of uric acid and the daily excretion of it. In most patients with primary hyperuricemia and gout, the fractional excretion of uric acid by the kidneys is relatively diminished, resulting in an imbalance of uric acid homeostasis, and serum urate exceeds saturability (6.8 mg/dL at physiologic temperature and pH). As this imbalance persists over years and decades, the miscible serum uric acid pool expands and urate may be deposited as part of an insoluble urate pool in the joints and soft tissues, referred to as tophi.
In the past, most investigators have focused on the destructive and proinflammatory role of insoluble deposited urate crystals, but new evidence is accumulating that rising levels of soluble urate in body fluids may also be harmful and lead to kidney disease, hypertension, and cardiovascular disease.
RENAL MANIFESTATIONS OF HYPERURICEMIA
Urate is strongly associated with renal disease but traditionally has not been considered to have a causal role in kidney dysfunction. The exceptions have been uric acid kidney stones and the acute uric acid nephropathy associated with chemotherapy and tumor lysis syndrome. New epidemiologic studies in humans, as well as an animal model of mild hyperuricemia leading to microvascular changes in the glomerular afferent arterioles, shed new light on a possible direct role of urate in the genesis of idiopathic chronic kidney disease.
Longstanding reluctance to implicate urate in kidney disease
Significant impairment of renal function was reported in up to 40% of patients with gout in studies conducted before the advent of effective urate-lowering therapies.3,4 In these older studies, renal failure was the eventual cause of death in 18% to 25% of patients with gout.3,4 However, any primary causal relationship for urate in this very high incidence of kidney disease was questioned for decades in light of the many other conditions and factors associated with hyperuricemia and gout that may contribute to kidney disease, such as hypertension, diabetes mellitus, alcohol abuse, non-steroidal anti-inflammatory drug use, and lead toxicity.
Recent epidemiologic studies establish the connection
More recently, two large prospective population studies from Japan examined the relationship between serum urate level and development of kidney disease using multiple covariate analysis to adjust for age, blood pressure, body mass index, proteinuria, hematocrit, hyperlipidemia, fasting glucose, and serum creatinine.5,6