Assessing benefits and risks of hormone therapy in 2008: New evidence, especially with regard to the heart
ABSTRACT
Observational studies, including the observational component of the Women's Health Initiative, consistently found that women who chose to use menopausal hormone therapy (HT) had a reduction in mortality and cardiovascular disease incidence relative to women who did not use HT. Randomized controlled trials have taught us that initiation of HT in older women (> 60 years old) remote from menopause (> 10 years since menopause) potentially has more risk than benefit. Additionally, randomized controlled trials have confirmed observational studies indicating the safety and benefit of HT in young (< 60 years old) recently menopausal women (< 10 years since menopause). In other words, we have come full circle in our understanding of HT, with a caveat concerning initiation in older women. Importantly, the magnitude and types of risk associated with HT are similar to those of other commonly used therapies. These data have led to recommendations that the benefits of HT exceed the risks when initiated in menopausal women younger than 60 years.
Duration of HT
Another hypothesis for the discordant findings between observational and randomized studies of HT focuses on differences in duration of HT use between the two types of studies. Duration of HT use has been substantially longer in observational studies, ranging from 10 years to 40 years, compared with no more than 7 or 8 years in randomized trials to date. Moreover, the results from observational studies have suggested that the longer the duration of HT use, the greater the benefit in terms of CHD risk.
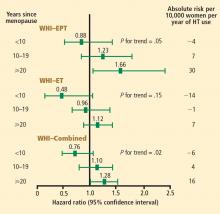
For example, a case-control study by Chilvers et al showed that HT protected against nonfatal myocardial infarction only when used for more than 60 months.27 Analysis of data on EPT use from the Heart and Estrogen/progestin Replacement Study (HERS),28 the randomized WHI E+P trial,20 and the WHI observational study9 reveals an interesting and consistent trend: rates of CHD events increased during the first year of EPT therapy (compared with placebo or nonuse) but then declined over time, ending up below the rates of CHD events in placebo recipients or nonusers of HT after approximately 5 years of therapy. This trend was not as pronounced with ET use in either the WHI randomized trial29 or the WHI observational study,10 but the incidence of CHD events did decline over time with ET use in both studies, and in the WHI observational study, greater than 5 years of ET use was associated with a significant 27% reduction (HR, 0.73; 95% CI, 0.61 to 0.84) in CHD events compared with nonuse.10
Timing of HT initiation
A third hypothesis for the discordance between randomized and observational studies of HT concerns the timing of HT initiation relative to patient age, time since menopause, and stage of atherosclerosis. As noted above, women enrolled in randomized trials have tended to be considerably older and further from menopause compared with their counterparts in observational studies. The effects of differences in timing of HT initiation on specific clinical end points, particularly in relation to cardiovascular health, are reviewed below.
Early and continued HT use may interrupt the pathogenic sequence of vascular aging, potentially preventing progression of atherosclerosis to the late stage at which plaque rupture and clinical events occur. In contrast, late intervention with HT may have little effect on established plaque and, in the case of EPT, may actually predispose to plaque rupture. This concept has been demonstrated in a pair of sister studies, the Women’s Estrogen-Progestin Lipid-Lowering Hormone Atherosclerosis Regression Trial (WELLHART)31 and the Estrogen Prevention Atherosclerosis Trial (EPAT).32
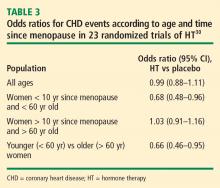
Mortality. The same Stanford University researchers who performed the above meta-analysis of CHD events also assessed odd ratios for overall mortality in a meta-analysis of 30 randomized trials of HT versus placebo that included a total of 26,708 postmenopausal women (representing 119,118 patient-years).33 They found the timing of HT initiation to have an effect on mortality similar to its effect on CHD events. In the overall cohort of women, the odds of death were not different between the HT and placebo groups, but among women younger than 60 years (mean, 54 years), those randomized to HT had a significant 39% reduction in the risk of death (HR = 0.61; 95% CI, 0.39 to 0.95) compared with those randomized to placebo. HT had no effect on mortality among women older than 60 years (mean, 66 years) in this analysis.
These mortality data are consistent with those from the WHI randomized trials, in which both EPT and ET were associated with a 30% reduction in overall mortality relative to placebo among women 50 to 59 years old.12 When both the EPT and ET portions of the WHI were combined to increase the sample size, HT was associated with a significant 30% reduction in mortality (HR = 0.70; 95% CI, 0.51 to 0.96) compared with placebo among women 50 to 59 years old.12
Stroke. The most recent WHI data indicate that stroke is not increased with ET in women 50 to 59 years of age, as there were 2 fewer events per 10,000 women per year of ET relative to placebo.12 In this same age group, the risk of stroke from EPT was increased by 5 events per 10,000 women per year of therapy relative to placebo.12 Among women randomized within 5 years of menopause, stroke risk was increased by 3 events per 10,000 women per year of EPT relative to placebo.13
VTE. Although age was not a significant contributing factor to the risk of VTE from HT in the WHI, the absolute risk of VTE was lower in younger versus older women. The additional absolute risk for VTE events per 10,000 women per year of EPT use was 11 events for women 50 to 59 years old at randomization, 16 events for women 60 to 69 years old at randomization, and 35 events for women 70 to 79 years old at randomization.15 The additional absolute risk for VTE events per 10,000 women per year of ET use was 4 events for women 50 to 59 years old at randomization, 7 events for women 60 to 69 years old at randomization, and 11 events for women 70 to 79 years old at randomization.23 A history of a prior thromboembolic event increases the risk of VTE with postmenopausal HT use, which should be considered before HT is initiated.