Prevention of venous thromboembolism in the cancer surgery patient
ABSTRACT
Cancer patients, especially those undergoing surgery for cancer, are at extremely high risk for developing venous thromboembolism (VTE), even with appropriate thromboprophylaxis. Anticoagulant prophylaxis in cancer surgery patients has reduced the incidence of VTE events by approximately one-half in placebo-controlled trials, and extended prophylaxis (for up to 1 month) has also significantly reduced out-of-hospital VTE events in clinical trials in this population. Clinical trials show no difference between low-molecular-weight heparin (LMWH) and unfractionated heparin in VTE prophylaxis efficacy or bleeding risk in this population, although the incidence of heparin-induced thrombocytopenia is lower with LMWH. The risk-benefit profile of low-dose anticoagulant prophylaxis appears to be favorable even in many cancer patients undergoing neurosurgery, for whom pharmacologic VTE prophylaxis has been controversial because of bleeding risks.
Venous thromboembolism (VTE) is a major complication of cancer, occurring in 4% to 20% of patients,1 and is one of the leading causes of death in cancer patients, although these figures are believed to be underestimates, given the low autopsy rates among cancer patients.2 In hospitalized cancer patients specifically, VTE is the second leading cause of death.3,4 The risk of VTE in cancer patients undergoing surgery is three to five times greater than that in surgical patients without cancer.4 Moreover, cancer patients with symptomatic deep vein thrombosis (DVT) exhibit a high risk of recurrent VTE that may persist for many years after the index event.5
VTE PREVENTION POSES PARTICULAR CHALLENGES IN CANCER PATIENTS
Until recently, data on VTE prevention specific to cancer patients have been sparse. Cancer patients have represented only a small subset (< 20%) of participants in most of the largest clinical trials of VTE prophylaxis. Until the past 2 or 3 years, clinicians largely have had to extrapolate their approach to VTE prophylaxis in cancer patients from data in patients without cancer, bearing in mind that cancer patients are among the populations at highest risk of developing VTE.
High rates of VTE, even with prophylaxis
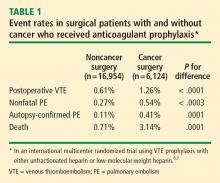
Further insights have come from the @RISTOS project, a Web-based prospective registry of patients undergoing general, urologic, or gynecologic surgery for cancer at multiple centers in Italy.8 Of the 2,372 patients tracked in this study, 82% received in-hospital VTE prophylaxis and 31% received prophylaxis following discharge. Despite this relatively high frequency of prophylaxis, however, the incidence of clinically overt VTE was 2.1% and the incidence of fatal VTE was 0.8%. Notably, most VTE events occurred after hospital discharge, and VTE was the most common cause of 30-day postoperative death in this registry.
RISK FACTORS: CANCER TYPE AND TREATMENT LOOM LARGE
Both the type and stage of a patient’s cancer are important in assessing the risk of VTE. For men, cancers of the prostate, colon, brain, and lung have been associated with an increased risk of VTE. Among women, cancers of the breast, ovary, and lung have been especially implicated as risk factors for VTE.9,10
The type of cancer therapy also influences VTE risk:
- Surgery. Among patients who undergo cancer-related surgery, the rate of proximal DVT is 10% to 20%, the rate of clinically evident PE is 4% to 10%, and the incidence of fatal PE is 0.2% to 5%.8,11
- Systemic treatments, including chemotherapy and hormone therapy, are also associated with an increased risk of VTE.12–15
- Central venous catheters. Approximately 4% of cancer patients who have central venous catheters placed develop clinically relevant VTE.16,17
In addition to the above risks related to cancer treatments, the following have been identified as risk factors for VTE in surgical oncology patients:
- Age greater than 40 years (risk also increases steeply after age 60 and again after age 75)
- Cancer procoagulants
- Thrombophilia
- Length and complications of cancer surgery (ie, often involving tissue trauma and immobilization)
- Debilitation and slow recovery.
Another risk factor worth noting is perioperative transfusion, as illustrated in a recent study of 14,104 adults undergoing colorectal cancer resection.18 The overall incidence of VTE in these patients was 1.0%, and the risk of death was nearly four times as great in patients who developed VTE as in those who did not. Notably, the need for transfusion was a marker of increased risk of VTE, particularly in women: women who received perioperative transfusions had almost double the risk of developing VTE compared with women who did not receive transfusions (P = .004).
CLINICAL TRIALS OF PROPHYLAXIS IN CANCER SURGERY PATIENTS
LMWH vs UFH for in-hospital prophylaxis
Two large randomized, double-blind trials have compared low-molecular-weight heparin (LMWH) with low-dose unfractionated heparin (UFH) for VTE prophylaxis in surgical patients with cancer—the Enoxaparin and Cancer (ENOXACAN) study19 and the Canadian Colorectal Surgery DVT Prophylaxis Trial.20 Patients in these studies underwent surgery for abdominal or pelvic cancer (mostly colorectal cancer). Both studies compared 40 mg of the LMWH enoxaparin given once daily with 5,000 U of UFH given three times daily for 7 to 10 days postoperatively. Outcome measures were the presence of DVT determined by venography on day 7 to 10 and the incidence of symptomatic VTE. Rates of VTE were statistically equivalent between the two treatment arms in both ENOXACAN (14.7% with LMWH vs 18.2% with UFH) and the Canadian Colorectal Surgery study (9.4% with both therapies), as were rates of major bleeding (4.1% with LMWH vs 2.9% with UFH in ENOXACAN; 2.7% with LMWH vs 1.5% with UFH in the Canadian study).
These findings are consistent with a 2001 meta-analysis by Mismetti et al of all available randomized trials comparing LMWH with placebo or with UFH for VTE prophylaxis in general surgery.21 This analysis found no differences in rates of asymptomatic DVT, clinical PE, clinical thromboembolism, death, major hemorrhage, total hemorrhage, wound hematoma, or need for transfusion between LMWH and UFH in patients undergoing either cancer-related surgery or surgery not related to cancer.
Fondaparinux for in-hospital prophylaxis
Subgroup analysis of the large randomized trial known as PEGASUS22 sheds some light on the efficacy of the factor Xa inhibitor fondaparinux relative to LMWH for thromboprophylaxis in cancer surgery patients. PEGASUS compared fondaparinux 2.5 mg once daily with the LMWH dalteparin 5,000 IU once daily for 5 to 9 days in patients undergoing high-risk abdominal surgery. Among the study’s 1,408 patients undergoing surgery for cancer, rates of VTE were 4.7% in the fondaparinux group compared with 7.7% in the LMWH group, a relative risk reduction of 38.6% with fondaparinux (95% CI, 6.7% to 59.6%). In contrast, in the rest of the PEGASUS population (patients undergoing abdominal surgery for reasons other than cancer), LMWH was nonsignificantly more efficacious at preventing VTE than was fondaparinux. Rates of major bleeding in this cancer subgroup were comparable between the two treatments.