Prevention of venous thromboembolism in the hospitalized medical patient
ABSTRACT
Hospitalized acutely ill medical patients are at high risk for venous thromboembolism (VTE), and clinical trials clearly demonstrate that pharmacologic prophylaxis of VTE for up to 14 days significantly reduces the incidence of VTE in this population. Guidelines recommend use of low-molecular-weight heparin (LMWH) or unfractionated heparin (5,000 U three times daily) for VTE prophylaxis in hospitalized medical patients with risk factors for VTE; in patients with contraindications to anticoagulants, mechanical prophylaxis is recommended. All hospitalized medical patients should be assessed for their risk of VTE at admission and daily thereafter, and those with reduced mobility and one or more other VTE risk factors are candidates for aggressive VTE prophylaxis. Based on results from the recently reported EXCLAIM trial, extended postdischarge prophylaxis with LMWH for 28 days should be considered for hospitalized medical patients with reduced mobility who are older than age 75 or have a cancer diagnosis or a history of VTE.
Population amended at planned interim analysis
After approximately half of the patients were enrolled, a planned and blinded interim analysis for futility concluded that the study was unlikely to show a statistically significant advantage of enoxaparin over placebo. The trial’s steering committee followed the suggestion of its data safety monitoring board to redefine the inclusion criteria to refocus enrollment on patients with a high risk of VTE. A blinded analysis was performed to identify this subgroup.
The resulting amended inclusion criteria were the same as above except that level 2 mobility had to be accompanied by at least one of three additional high-risk criteria: (1) age greater than 75 years, (2) history of prior VTE, or (3) diagnosis of cancer.
The trial’s main exclusion criteria were evidence of active bleeding, a contraindication to anticoagulation, receipt of prophylactic LMWH or UFH more than 72 hours prior to enrollment, treatment with an oral anticoagulant within 72 hours of enrollment, major surgery within the prior 3 months, cerebral stroke with bleeding, and persistent renal failure (creatinine clearance < 30 mL/min).


Results
The amended study population included 5,105 patients, 5,049 of whom received open-label enoxaparin. Of this group, 2,013 were randomized to active extended prophylaxis with enoxparain and 2,027 to placebo. Baseline characteristics, including level of mobility, were similar between the two groups.
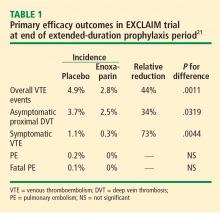
The efficacy of extended prophylaxis with enoxaparin was enduring, as the cumulative incidence of VTE events at day 90 was significantly lower in enoxaparin recipients than in placebo recipients (3.0% vs 5.2%; relative reduction of 42%; P = .0115).
There was no difference in all-cause mortality at 6 months between the enoxaparin and placebo groups (10.1% vs 8.9%, respectively; P = .179).
Safety. Major hemorrhage was significantly more frequent in the enoxaparin arm, occurring in 0.60% of enoxaparin recipients compared with 0.15% of placebo recipients (P = .019). Minor bleeding was also more common with enoxaparin (5.20% vs 3.70%; P = .024).
Conclusions
The EXCLAIM trial found that an extended-duration (38-day) enoxaparin regimen significantly reduced the overall incidence of VTE relative to a 10-day enoxaparin regimen in acutely ill medical patients with reduced mobility. At the same time, the extended regimen was associated with a significant increase in the rate of major bleeding, although the incidence of major bleeding was low. The investigators concluded that the net clinical effect of extended-duration prophylaxis with enoxaparin is favorable, as only 46 patients would need to be treated to prevent one VTE event, whereas 224 patients would need to be treated to result in one major bleeding event.21
For this reason, it is reasonable to consider extended prophylaxis for hospitalized medical patients after identifying these patients’ risk factors. In keeping with the trial’s amended inclusion criteria, patients older than age 75 and those with cancer or prior VTE should receive special consideration for extended prophylaxis.
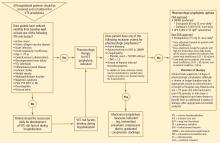
RECOMMENDED APPROACH TO VTE PREVENTION IN HOSPITALIZED MEDICAL PATIENTS
- All hospitalized medical patients should be screened at the time of admission, and patients at risk for VTE should receive prophylaxis.
- All patients with reduced mobility and one or more other risk factors for VTE are candidates for prophylaxis.
- Patients should be reassessed daily for the development of VTE risk factors during their hospitalization if risk factors are absent on admission.
- If screening or reassessment reveals any VTE risk factors, pharmacologic prophylaxis is the mainstay of therapy. If exclusion criteria for pharmacologic prophylaxis are present, mechanical prophylaxis with graduated compression stockings and intermittent compression devices should be used. For very high-risk medical patients without a contraindication to anticoagulants, combination prophylaxis with both an anticoagulant and mechanical devices is preferred.
- In this patient population, LMWH agents are preferred as pharmacologic prophylaxis over UFH and over fondaparinux (which is not currently approved by the US Food and Drug Administration for this population).
- If UFH is to be used in this patient population, 5,000 U three times daily is the preferred dosage.
- Extended pharmacologic prophylaxis should be considered in patients older than age 75 and in patients with a cancer diagnosis or a prior VTE episode.