Heart-brain medicine: Update 2007
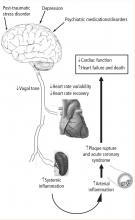
Heart-brain medicine is dedicated to furthering our understanding of the interaction between the body’s neurologic and cardiovascular systems. As discussed previously,1 the advent of subspecialization in health care delivery has led to significant advances in the care of patients with acute disease or acute exacerbations of chronic disease. While these advances have led to improved outcomes, we were reminded several times this past year how difficult it is to further improve outcomes using the “silo”-based, highly subspecialized approach that has yielded results in the past.
The 2007 Bakken Heart-Brain Summit, held last June in Cleveland, further demonstrated real progress in our understanding of the importance of heart-brain interactions in health and disease. A series of presentations—highlighted by the Bakken Lecture given by Peter Shapiro, MD, an investigator with the SADHART trial—reviewed the effect of psychiatric disorders on the incidence of cardiovascular disease and its consequences. These presentations by leaders in the field (many of which are summarized in the pages that follow) offer irrefutable evidence of the following:
- Patients with depression and heart disease have worse outcomes than patients with heart disease without depression2
- Patients with depression have decreased vagal tone3
- Patients with coronary artery disease (CAD) can be safely treated with and respond to antidepressants.4
These data were complemented by a keynote presentation by Kevin Tracey, MD, whose elegant work over the past many years has demonstrated a link between vagal tone and inflammation.5 His most recent data have shown that the vagus has direct input into the inflammatory state of macrophages in the spleen. The effect is mediated via vagal innervation of the spleen and the α7 subunit of the nicotinic receptor expressed on the cell surface of the resident macrophages.6,7 The relevance of vagally mediated modulation of systemic inflammation has been shown in sepsis and more recently by our group in left ventricular remodeling following acute myocardial infarction.
‘RECONNECTING THE BODY’ TO IMPROVE OUTCOMES
The continuing emergence of the link between psychiatric and neurocontrol of systemic inflammation offers an undeveloped strategy for further improving outcomes in patients with cardiovascular disease. One of our interests in pursuing heart-brain medicine is to reconnect the body and exploit the physiologic interplay between the heart and brain to improve patient outcomes.1 Given the disappointments over the past year for new therapies like cholesteryl ester transfer protein inhibitors8 and vascular cell adhesion molecule (VCAM) inhibitors, strategies that have a singular organ or cellular target focus, now may be the time for exploiting multisystem approaches for modulating disease states such as CAD, congestive heart failure, and arrhythmia.
The potential consequences of these pathways are profound and include the following:
- A physiologic mechanism for the increased incidence of myocardial infarction observed with medications that have anticholinergic properties and potentially decrease autonomic tone
- Worse outcomes in patients with CAD and depression
- An increased incidence of CAD in patients with psychiatric disorders that in themselves may be associated with decreased vagal tone, as well as in patients on long-term drug therapies that alter parasympathetic tone
- Increased incidences of CAD, myocardial infarction, and death in patients with PTSD.