Sudden unexpected death in epilepsy: Impact, mechanisms, and prevention
ABSTRACT
Patients with refractory epilepsy face an elevated risk of sudden death, with rates as high as 1% per year. This phenomenon, known as sudden unexpected death in epilepsy (SUDEP), is believed to be a seizure-related occurrence, but the exact underlying mechanisms are uncertain. Both pulmonary and cardiac pathophysiologies have been proposed. The cardiac mechanism of greatest interest is the precipitation of arrhythmias by seizure discharges via the autonomic nervous system. SUDEP prevention has centered on effective seizure control, and epilepsy surgery has reduced SUDEP incidence in a number of studies. Additional prophylaxis methods are needed, however, for the large number of patients with treatment-refractory epilepsy. Future research should aim to clarify whether the association between seizures and autonomic dysfunction and cardiac arrhythmias extends to a demonstrable cardiac mechanism for SUDEP.
The intimate interplay between heart and brain is well illustrated in epilepsy and may underlie the mechanism of one of its most devastating consequences: sudden unexpected death in epilepsy (SUDEP). This article will briefly describe the potential mechanisms of SUDEP, elaborate on the evidence for a likely cardiac pathophysiology, and review considerations in SUDEP prevention. We begin with a couple of brief case presentations and an epidemiologic overview to illustrate the concept and significance of SUDEP.
CASE PRESENTATIONS
A patient with near-SUDEP
The following is an actual message received by one of the authors:
Dr. Najm: A quick note regarding a 27-year-old male patient of yours with cerebral palsy and seizure disorder. Yesterday, while being transported from floor to floor, he had a cardiac arrest and was successfully resuscitated. Immediately after the code he developed seizures, which were treated with phenytoin and lorazepam. He is now in the neurointensive care unit. Thank you.
This case represents a scenario of near-SUDEP in which death was prevented by the fortuitous presence of immediate medical assistance at the time of cardiac arrest. Had this patient been home at the time of this incident, he almost certainly would have simply been found dead in his bed, like many SUDEP victims.
A typical case with multiple risk factors
A 32-year-old man underwent left temporal lobectomy at the Cleveland Clinic for treatment of medically refractory focal epilepsy. His seizure frequency improved after surgery, but he continued to have rare convulsions. Nevertheless, he discontinued all his anticonvulsant medications on his own. One year later, he was found dead on his bathroom floor. No obvious cause of death was identified.
This case illustrates several characteristics of the patient typically at risk for SUDEP: young, male, with intractable poorly controlled epilepsy, and not taking antiepileptic medications.
EPIDEMIOLOGY AND RISK FACTORS
Epilepsy affects 1% of the US population. Among those affected by epilepsy, SUDEP is a common cause of mortality. Estimates of SUDEP incidence range from 0.7 to 1.3 cases per 1,000 patient-years in large cohorts of patients with epilepsy1,2 and from 3.5 to 9.3 cases per 1,000 patient-years in anticonvulsant drug registries, medical device registries, and epilepsy surgery programs.3–5 SUDEP accounts for up to 17% of all deaths in patients with epilepsy6,7 and exceeds the expected rate of sudden death in the general population by nearly 24 times.6,8
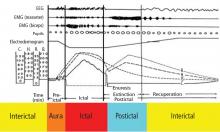
Several potential risk factors for SUDEP have been investigated, but results from different studies are conflicting. Consistently identified risk factors include young age, early onset of seizures, refractoriness of epilepsy, the presence of generalized tonic-clonic seizures, male sex, and being in bed at the time of death. Weaker risk factors include being in the prone position at the time of death, having one or more subtherapeutic blood levels of anticonvulsant medication, having a structural brain lesion, and being asleep.9 The current consensus is that SUDEP is primarily a “seizure-related” occurrence, but the exact mechanisms underlying SUDEP are unknown.
PROPOSED MECHANISMS
Pulmonary pathophysiology
Central apnea and acute neurogenic pulmonary edema are the two major proposed pathways linking seizures to SUDEP. Evidence exists for each pathway.
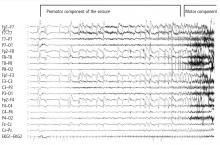
Central apnea. In a prospective study of patients in an epilepsy monitoring unit, central apnea lasting at least 10 seconds was observed postictally in 40% of the recorded seizures.10 Otherwise healthy young epilepsy patients have been reported to develop central apnea immediately following complex partial seizures.11 Neurotransmitters mediating the brain’s own seizure-terminating mechanism could also be inhibiting the brainstem and causing postictal apnea.
Acute neurogenic pulmonary edema has been well described in relation to severe head injury and subarachnoid hemorrhage. Pulmonary edema is frequently found in SUDEP patients at autopsy.12 Intense generalized vasoconstriction induced by massive seizure-related sympathetic outburst can lead to increased pulmonary vascular resistance, and thereby may mediate acute pulmonary edema.
These two mechanisms—central apnea and acute neurogenic pulmonary edema—are not mutually exclusive. In the only animal model of SUDEP, one third of animals died from hypoventilation and had associated pulmonary edema at autopsy.13 Limited opportunities for realistic and practical interventions to reverse SUDEP risks related to pulmonary causes have hindered further development of these concepts.
Cardiac pathophysiology
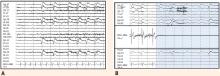
The most significant and widely discussed cardiac mechanism of SUDEP is cardiac arrhythmia precipitated by seizure discharges acting via the autonomic nervous system.14–19
Experimental evidence. Heart rate changes, including bradycardia, tachycardia, and even asystole, have been repeatedly provoked by electrical brain stimulation of the limbic system and insular cortex.19 Some studies have suggested a lateralized influence of the insulae on cardiovascular autonomic control. In one study, intraoperative stimulation of the left posterior insula elicited a cardioinhibitory response and hypotension, whereas stimulation of the right anterior insula elicited tachycardia and hypertension.20 Such results have not always been reproducible.21–23 Other studies have suggested a localization-related influence of the limbic system on cardiovascular responses. Stimulation of the amygdala has not led to the ictal tachycardia that is commonly seen in epileptic seizures, suggesting that cortical involvement is needed for the development of tachycardia.24