Deep brain stimulation: How does it work?
ABSTRACT
Deep brain stimulation has significantly improved the motor symptoms in patients with Parkinson's disease (PD) and other movement disorders. The mechanisms responsible for these improvements continue to be explored. Inhibition at the site of stimulation has been the prevailing explanation for the symptom improvement observed with deep brain stimulation. Research using microelectrode recording during deep brain stimulation in the MPTP monkey model of PD has helped clarify how electrical stimulation of structures within the basal ganglia-thalamocortical circuit improves motor symptoms, and suggests that activation of output and the resultant change in pattern of neuronal activity that permeates throughout the basal ganglia motor circuit is the mechanism responsible for symptom improvement.
Whether deep brain stimulation can dramatically help patients with Parkinson’s disease (PD) and other movement disorders is no longer questioned. Rather, how it works is not well understood: how do patients with seemingly diverse conditions show improvement with the same intervention?
Patients with advanced PD often freeze when trying to walk and have tremor, rigidity, bradykinesia, and gait and balance problems. With deep brain stimulation, a patient typically experiences a marked improvement in these motor symptoms.
Similarly, patients with hypokinetic disorders such as generalized dystonia who have extensive involuntary movements involving multiple body parts may experience a significant reduction in these movements and regain function during deep brain stimulation. In my experience, it is not unusual for patients who were not ambulatory as a result of their dystonic movements to regain function to the point where they can walk unassisted and, in some cases, participate in physical activities such as racquetball or jogging on a treadmill. One of my patients with generalized dystonia could walk no farther than several meters before deep brain stimulation but afterward was able to run on a treadmill. This patient did not gain this type of function immediately after stimulation, but after sustained efforts at programming his stimulation device over the course of 1 year he was able to travel to Europe, hike in the mountains, and jog on a treadmill.
In addition to treating movement disorders, deep brain stimulation is being used experimentally to treat patients with behavioral disorders such as depression and obsessive-compulsive disorder that are refractive to standard therapy. Broadening our understanding of the mechanisms responsible for success with deep brain stimulation is important since it may help to improve current applications and develop new ones. This article discusses our research in deep brain stimulation using microelectrode recording of structures within the basal ganglia–thalamocortical circuit in the MPTP monkey model of PD.
INSIGHTS INTO MECHANISMS OF STIMULATION PROMISE TECHNOLOGICAL REFINEMENTS
One rationale for attempting to better understand how deep brain stimulation works is that such knowledge may enable us to improve the technology to better apply the technique.
Electrode design is one important area of potential improvement. Diseases that may one day be treated with deep brain stimulation will likely require electrodes of different shapes than those used currently, to accommodate other targets in the brain. At present, a single lead shape is used to stimulate the subthalamic nucleus (STN) and the globus pallidus internus (GPi) for treating PD. Possible future targets include the globus pallidus externus (GPe), various subnuclei of the thalamus, portions of the striatum, and other subcortical and cortical structures that have different geometric configurations and physiologic characteristics. Since these structures and regions of the brain differ from one another in size and shape, it is highly likely that new electrode designs will be needed to take advantage of this geometric and physiologic variability. Future electrodes may vary in size and shape from those used currently, incorporate three-dimensional designs, and require a current source that allows the pattern of stimulation to be varied based on the physiologic changes that characterize each neurologic disorder.
Directionality may be another important feature of electrode design. With presently used electrodes, electric current spreads in all directions. To spread the current or increase the volume of tissue affected by stimulation, one must increase the voltage being passed through the lead. This results in a larger region of tissue being affected by stimulation, but the current density varies based on distance from the stimulation site, with neural tissue close to the site being affected differently from tissue that is farther away. Moreover, the current cannot be directed or aimed in one direction or the other. A split-band design could spread current in opposing directions, and a three-dimensional directional design involving several contacts could affect a volume of tissue more homogeneously.
PROGRESS IN DEFINING PD PATHOPHYSIOLOGY
As with any disease, defining the problem and understanding the underlying pathophysiology are essential first steps to finding an effective treatment for PD. In the 1930s and 1940s, numerous attempts were made to treat PD with surgical therapies. Surgical targets were chosen throughout the length of the neuraxis, including the cortex, the internal capsule, the basal ganglia, the thalamus, the cerebral peduncle, and the spinal cord itself. The underlying pathophysiology was not well understood, however, so the rationale for surgery was weak at best. For example, lesioning the cortex improved parkinsonian tremor, but it also caused paralysis and was associated with considerable morbidity.
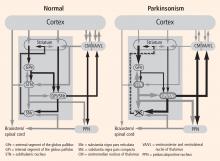
Evidence of a common circuit
In PD, degeneration of dopamine-producing neurons in the substantia nigra pars compacta reduces dopamine levels in the striatum. In MPTP monkey models of PD there is also a loss of dopamine-producing cells in the substantia nigra pars compacta. These animals develop the cardinal motor symptoms of PD and are considered a good model of the human disorder. By recording from the basal ganglia–thalamocortical circuit in this model, we and others have observed excessive activity in the STN and GPi.1–4 In addition, cells in these regions in the monkey model were more likely to discharge in bursts compared with cells from healthy monkeys, and they showed a higher degree of synchronized oscillatory activity among neighboring neurons.5,6
Ultimate goal: The ability to individualize therapy
Understanding how such changes relate to parkinsonian symptoms will enable us to develop stimulation strategies that are focused on ameliorating the particular physiologic changes in PD. Since PD can lead to distinctly different clinical pictures, it would be ideal to be able to individualize therapy based on the particular motor symptoms each patient experiences. This may require stimulation strategies that affect either a particular region of the targeted structure or a particular physiologic change that occurs in the disease state.