New asthma guidelines emphasize control, regular monitoring
ABSTRACTUpdated asthma care guidelines have recently been released. This review will focus on several elements in the third Expert Panel Report (EPR3) guidelines that reflect substantial differences from recommendations of the second EPR (EPR2) guidelines, issued in 1997 and updated in 2002. A major difference is the emphasis on asthma control. Asthma control can be assessed serially by the use of validated instruments. The goal of asthma therapy is to achieve asthma control by reducing current impairment and future risk. Recommendations for asthma pharmacotherapy have also been revised since the release of the updated EPR2 guidelines. The revisions in asthma management proposed in these guidelines offer the potential for improved asthma care outcomes in the United States.
KEY POINTS
- The EPR3 recommends that management decisions be based initially on asthma severity, and subsequently on asthma control as assessed serially by validated tests.
- Omalizumab, a monoclonal antibody against immunoglobulin E, is the only adjunctive therapy to demonstrate efficacy when added to high-dose inhaled corticosteroids plus long-acting beta agonists in patients with severe, persistent, allergic asthma.
- The EPR3 guidelines recommend consideration of allergen immunotherapy for patients with mild or moderate persistent allergic asthma.
Validated tests are available to assess control
Serial testing as a quality indicator
Serial ACT scores give an objective measure of the degree to which the goals of management1 are being achieved, and in so doing can encourage optimal outcomes.14
Another use of these tests is to document whether asthma control improves over time when patients receive care from a particular physician or group. This use may become increasingly important in view of efforts underway to implement a pay-for-performance model for asthma care, in which providers will be financially rewarded for improved patient care outcomes and adherence to standards of practice based on Health Plan Employer Data and Information Set measures.15
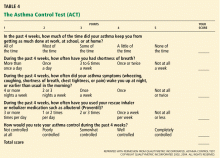
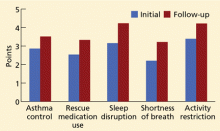
We have used the ACT in the Section of Allergy/Immunology at Cleveland Clinic for 3 years on a routine basis. All patients with asthma being seen either for the first time or as follow-up complete the ACT, which has been entered in a flow sheet in our electronic medical record, at the same time they undergo spirometry. We have shown that care in the Section of Allergy/Immunology is associated with improvement in asthma control over time, in patients who have completed serial ACT measurements at initial visits and at follow-up visits (Figure 2).
Objective measurement of lung function is also important
Serial monitoring of lung function at every patient visit with spirometry is also important, as some patients may be “poor perceivers,”16 ie, they may have little or no subjective awareness of moderate or even severe ventilatory impairment. A number of studies17,18 support the contention that symptoms and lung function are separate and independent dimensions of asthma control, and that both of them need to be assessed.
Responding to changes in control
THE STEP 3 CONTROVERSY
Salmeterol Multicenter Asthma Research Trial
In the Salmeterol Multicenter Asthma Research Trial (SMART), patients randomized to the long-acting beta agonist (LABA) salmeterol (Serevent)—particularly African Americans—had a statistically significant increase in the risk of untoward asthma care outcomes.20
SMART was launched in 1996. Patients were randomized in a double-blind fashion to receive either salmeterol 42 μg twice a day or placebo in addition to their usual asthma therapy for 28 weeks. The rate of the primary outcome (respiratory-related deaths or life-threatening experiences) was not significantly different with salmeterol than with placebo (relative risk [RR] = 1.40, 95% confidence interval [CI] 0.91–2.14). However, in 2003, the study was halted prematurely because of difficulty enrolling the targeted number of 60,000 patients, and an interim analysis that revealed significantly higher rates of secondary outcomes in subjects randomized to salmeterol. Compared with the placebo group, the salmeterol group had significantly higher rates of respiratory-related deaths (RR 2.16, 95% CI 1.06–4.41), asthma-related deaths (RR = 4.37, 95% CI = 1.25–15.34), and combined asthma-related deaths or life-threatening experiences (RR = 1.71, 95% CI 1.01–2.89). There were 13 asthma-related deaths and 37 combined asthma-related deaths or life-threatening experiences in the salmeterol group, compared with 3 and 22, respectively, in the placebo group. Of the 16 asthma deaths in the study, 13 (81%) occurred in the initial phase of SMART, when patients were recruited via print, radio, and television advertising; afterward, patients were recruited directly by investigators.
Statistically significant differences in outcomes occurred primarily in African Americans. African Americans who received salmeterol had higher rates of respiratory death or life-threatening experiences (RR = 4.10, 95% CI 1.54–10.90), the primary end point for the study, as well as higher rates of combined asthma-related deaths or life-threatening experiences (RR = 10.46, 95% CI 1.34–81.58), a secondary end point. No statistically significant differences were observed in white patients randomized to salmeterol with respect to the primary end point (RR = 1.05, 95% = 0.62–1.76); the secondary end point of combined asthma-related deaths or life-threatening experiences (RR = 1.08, 95% CI 0.55–2.14); or other end points.
Medication exposures were not tracked during the study, and allocation to inhaled corticosteroids combined with salmeterol was not randomized, so the effect of concomitant inhaled corticosteroid use cannot be determined from these data.
As a result of SMART, medications that contain either of the two LABAs, salmeterol or formoterol (Foradil), carry a black-box warning.