Given the ENHANCE trial results, ezetimibe is still unproven
Ezetimibe (Zetia) was licensed by the US Food and Drug Administration in 2002 on the basis of its ability to reduce low-density lipoprotein cholesterol (LDL-C) levels. The reductions are mild, approximately 15%,1 which is comparable to the effects of a stringent diet and exercise or of a statin in titrated doses.
However, there was no evidence that ezetimbe, which has a unique mechanism of action, delivers a benefit in terms of clinical outcomes. Despite this, the use of ezetimibe (alone or in fixed-dose combination with simvastatin, a preparation sold as Vytorin) grew rapidly, generating annual sales of $5.2 billion. Clinicians and the manufacturer (Merck/Schering-Plough) broadly assumed that LDL-C reduction would carry ezetimibe’s day as clinical trials emerged.
The assumption seemed reasonable, since evidence from the past 3 decades has established a clear link between lowering LDL-C levels via diverse mechanisms and positive clinical outcomes, particularly lower rates of cardiovascular disease and death. Indeed, LDL-C measurement is now a focus of cardiovascular risk assessment and management, as reflected in national treatment guidelines.
THE ENHANCE TRIAL: EZETIMIBE FAILS A KEY TEST
Unexpectedly, ezetimibe failed its first step in clinical trial validation, the Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) trial.2 Apart from the scientifically irrelevant political regulatory intrigue generated by the sponsor’s conduct in this trial, ENHANCE’s findings challenge us to confront issues of what we assume vs what we really know, and how to interpret the complex results of clinical trials.
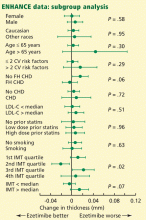
To be fair to the trial’s investigators, ENHANCE achieved its objective of enrolling a population with a very high LDL-C level, which is ezetimibe’s target and has been widely used in the study of atherosclerosis progression as a marker of potential drug benefit. Nevertheless, and even though the LDL-C level 2 years later was 52 mg/dL lower in the group receiving ezetimibe/simvastatin than in the group receiving simvastatin alone (Zocor), at LDL-C levels that are typically associated with atherosclerosis progression (140–190 mg/dL), ezetimibe failed to reduce the progression of atherosclerosis.
These trends are particularly worrisome, given that the ezetimibe/simvastatin group achieved a greater reduction in C-reactive protein levels, which typically has resulted in superior outcomes in atherosclerosis3 and clinical effects4 in combination with LDL-C reduction.
In view of these findings, should clinicians stand firm and continue to use ezetimibe? Or should we reevaluate our position and await more data about this unique, first-in-class compound?
WISHFUL POST HOC HYPOTHESES
In this issue of the Cleveland Clinic Journal of Medicine, Dr. Michael Davidson,5 a respected lipid expert but one invested in ezetimibe’s development, assures us that all is in order and that the results of ENHANCE can be explained away by several arguments, most notably that most of the trial’s participants had previously received lipid-lowering treatment, which obscured the effects of ezetimibe. Moreover, he argues that ezetimibe’s mechanism of action is well understood and that the drug is safe and well tolerated and thus should remain a first-line treatment for hyperlipidemia.
These arguments may eventually prove to be correct, but as of now they are merely wishful post hoc hypotheses awaiting more data apart from ENHANCE. Negative clinical trials do occur as a matter of chance, but we should be cautious in any attempts to explain away a trial that was designed, executed, and reported as conceived simply because the results do not match our expectations.
Confronted with ENHANCE, the astute clinician should ask three questions: Do we really understand ezetimibe’s mechanism of action? Do other lines of evidence indicate the drug is beneficial? And how reliable is the arterial thickness as a surrogate end point?