A young woman with fatigue
CONFIRMING CELIAC DISEASE
2. Which of the following is used to test for celiac disease?
- Immunoglobulin G (IgG) and immunoglobulin A (IgA) antigliadin antibody testing
- IgA antiendomysial antibody and IgA antitransglutaminase antibody testing
- HLA DQ2/DQ8 testing
The sensitivity of antigliadin antibody testing is only about 70% to 85%, and its specificity is about 70% to 90%. Better serologic tests are those for IgA antiendomysial and antitransglutaminase antibodies, which have sensitivities greater than 90% and specificities greater than 95%.3 HLA DQ2/DQ8 testing has a high sensitivity (> 90%–95%), but because about 30% of the general population also carry these markers, the specificity of this test is not ideal. This test is best used for its negative predictive value—ie, to rule out the diagnosis of celiac disease.
Of note: 1% to 2% of patients with celiac disease have a deficiency of IgA.4 Therefore, if the clinical suspicion for celiac disease is high but the IgA antibody tests are negative or equivocal, IgG antitransglutaminase and IgG antiendomysial antibody tests can help establish the diagnosis. HLA testing in this situation can also help rule out the diagnosis.
CONFIRMING CELIAC DISEASE—CONTINUED
3. What test should be performed next in this patient?
- Upper GI series with small-bowel follow-through
- Esophagogastroduodenoscopy with biopsies
- Small-bowel barium study
- Video capsule endoscopy
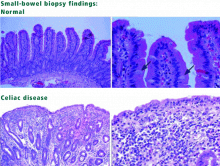
Today, the presumptive diagnosis of celiac disease requires positive serologic testing and biopsy results. Esophagogastroduodenoscopy with biopsies should be ordered. Upper GI series and barium studies do not provide a tissue diagnosis. Barium studies and other radiologic tests can be considered if a patient does not have the expected response to a strict gluten-free diet or if one suspects complications of celiac disease, such as GI lymphoma.
Video capsule endoscopy is an emerging tool for diagnosing celiac disease, as suggested in several trials.5 Some findings seen on video capsule endoscopy in patients with celiac disease include mosaicism, nodularity, visible vessels, and loss of mucosal folds. However, the role of this test continues to be investigated, and biopsy is still required to confirm the diagnosis.
WHO SHOULD BE TESTED FOR CELIAC DISEASE?
The reported prevalence of symptomatic celiac disease is about 1 in 1,000 live births in populations of northern European ancestry, ranging from 1 in 250 (in Sweden) to 1 in 4,000 (in Denmark).6 The prevalence appears to be higher in women than in men.7
In a large US study, the prevalence of celiac disease was 1 in 22 in first-degree relatives of celiac patients, 1 in 39 in second-degree relatives, 1 in 56 in patients with either GI symptoms or a condition associated with celiac disease, and 1 in 133 in groups not at risk.8 Another study found that the prevalence of antiendomysial antibodies in US blood donors was as high as 1 in 2,502.
Given that patients with celiac disease may not present with classic symptoms, it has been suggested that the following groups of patients be tested for it1:
- Patients with GI symptoms such as chronic diarrhea, malabsorption, weight loss, or abdominal symptoms
- Patients without diarrhea but with other unexplained signs or symptoms that could be due to celiac disease, such as iron-deficiency anemia, elevated aminotransferase levels, short stature, delayed puberty, or infertility
- Symptomatic patients at high risk for celiac disease. Risk factors include type 1 diabetes or other autoimmune endocrinopathies, first- and second-degree relatives of people with celiac disease, and patients with Turner, Down, or Williams syndromes.
Screening of the general population is not recommended, even in populations at high risk (eg, white people of northern European ancestry).