Nonalcoholic fatty liver disease: A manifestation of the metabolic syndrome
ABSTRACTNonalcoholic fatty liver disease (NAFLD) has become the most common form of liver disease, affecting 20% to 30% of the US population. Its clinical manifestations are usually absent or subtle, and it usually comes to medical attention incidentally when aminotransferase levels are found to be elevated or a radiographic study reveals that the liver is fatty. Primary NAFLD is now considered the hepatic manifestation of the metabolic syndrome. The pathogenesis is thought to be a multiple-hit process involving insulin resistance, oxidative stress, apoptosis, and adipokines. In general, the prognosis for simple steatosis is very good; however, nonalcoholic steatohepatitis (NASH) can progress to cirrhosis and hepatocellular carcinoma in 10% to 15% of patients. There is no established treatment for NAFLD except for weight loss and treating each component of the metabolic syndrome.
KEY POINTS
- The clinical spectrum of NAFLD ranges from simple steatosis to nonalcoholic steatohepatitis, cirrhosis, and hepatocellular carcinoma.
- NAFLD is closely associated with metabolic syndrome, insulin resistance, and obesity.
- Weight loss and treating components of the metabolic syndrome are central to the treatment of NAFLD. Insulin sensitizers such as biguanides and glitazones, antioxidants such as vitamin E, and lipid-lowering agents have shown promise in small clinical trials, but the evidence remains preliminary.
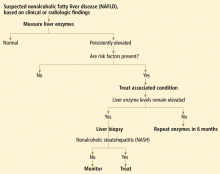
A DIAGNOSIS OF EXCLUSION
Excessive alcohol consumption must especially be excluded. Most studies defined excessive alcohol consumption as more than 20 to 40 g/day.2 Recently, this threshold has been lowered to 20 g/day (roughly two drinks) in men and 10 g/day in women.
Insulin resistance should be estimated, given the close relationship between NAFLD and insulin resistance and the metabolic syndrome. Insulin resistance can be measured accurately in a number of ways. The Homeostasis Model Assessment is an easy method that provides an estimate of insulin resistance based on fasting serum glucose and serum insulin levels.13
Serologic tests can rule out hepatitis B and hepatitis C. In those with negative results, especially in those with components of the metabolic syndrome or insulin resistance, NAFLD is responsible for most cases of persistently elevated serum liver enzymes.
Imaging tests
Radiographic evaluation is another noninvasive way to diagnose fatty liver. The sensitivity of either ultrasonography or computed tomography for detecting hepatic steatosis is between 93% and 100% when there is more than 33% fat in the hepatic parenchyma.14 None of the radiographic methods, including magnetic resonance imaging, can accurately differentiate between nonprogressive simple steatosis and NASH, but the technology is advancing. Contrast ultrasonography and magnetic resonance spectroscopy have shown promise and may become useful in the future.
Other noninvasive tests
Ultrasonographic elastrography (FibroScan), a noninvasive way to measure liver stiffness, has also been used in patients with hepatitis C. Although the preliminary data in NAFLD are interesting, additional validation is needed.
Serum biomarkers, including markers of fibrosis (eg, FibroSURE), apoptosis, and adipocytokines have been used to diagnose NASH. The markers of apoptosis are especially interesting but need further validation.
Liver biopsy remains the gold standard
Because we lack a fully validated noninvasive biomarker of NASH, liver biopsy remains the gold standard for diagnosing it. The minimum histologic criteria for establishing the diagnosis of NASH have been debated; most pathologists require at least 5% hepatic steatosis, mixed lobular inflammation, and hepatocellular ballooning.
In a study of 354 liver biopsies of patients with negative results on serologic tests, NASH was found in 34% and fatty liver in 32%. In the same study, the findings on liver biopsy led to alterations in patient management in 18% of cases.15
Some clinicians doubt the value of liver biopsy in patients with suspected NASH, in view of possible sampling error in the biopsy specimens (the distribution can be patchy, and if the specimen is taken from an unaffected area, the results can be falsely negative) and because there is no established effective therapy for NAFLD. However, liver biopsy is the only test that can accurately establish the diagnosis of NASH and tell us the stage of liver disease, which has important prognostic implications. Most experts agree that liver biopsy should be considered for patients at risk of advanced liver disease, such as those with persistently elevated liver enzyme levels despite intervention to reverse conditions associated with metabolic syndrome.16
PATHOGENESIS: THE MULTIPLE-HIT HYPOTHESIS
NAFLD is closely linked to obesity, insulin resistance, and metabolic syndrome.13 Insulin allows free fatty esterification and triglyceride fat storage in adipose tissues. When insulin resistance develops, free fatty acids are inappropriately shifted to nonadipose tissues, including the liver. Insulin resistance increases free fatty acid flux to the liver by decreased inhibition of lipolysis and also increased de novo lipogenesis.17
Insulin resistance and visceral obesity also result in decreased levels of a “protective adipokine,” adiponectin. Adiponectin inhibits liver gluconeogenesis and suppresses lipogenesis. Thus, decreased adiponectin hinders fatty acid oxidation and increases fat accumulation in the liver. Other adipocytokines that are important in NAFLD are resistin, leptin, visfatin, tumor necrosis factor alpha, and interleukin 6.
Apoptosis and oxidative stress may also contribute to the development and progression of NASH. In this context, the “multiple-hit hypothesis” for the pathogenesis of NASH has become quite popular.18 An in-depth review of the pathogenesis of NAFLD is beyond the scope of this paper; readers are referred to a recently published review on this subject.19
STEATOSIS IS BENIGN, BUT NASH CAN PROGRESS
Simple steatosis by itself generally has a benign prognosis. In a 1995 cohort study with a median follow-up of 11 years, there was no progression of simple steatosis to NASH or cirrhosis,20 and recent reviews estimate that only a small portion of patients with simple steatosis develop steatohepatitis. The validity of these data is still being debated.
On the other hand, once patients have progressed to NASH, histologic progression has been noted in about 32% to 41% of patients over a median follow-up of 4.3 to 13.7 years.21,22 This would mean that approximately 9% of patients with NASH may develop cirrhosis.21
People with cirrhosis due to NAFLD are at risk of developing liver-related morbidity and of death. In one of the longest follow-up cohort studies (mean follow-up of 13.7 years), end-stage liver disease developed in 5.4%, and hepatocellular carcinoma developed in about 2%. About 20% of the patients died, with more than 70% of the deaths in patients who had NASH at baseline. The survival rate was lower in patients with NASH, whereas no difference in survival was seen in the group with simple steatosis.22
A number of studies have assessed independent predictors of advanced fibrosis. Most studies suggest that elevated liver enzymes, metabolic syndrome, or type 2 diabetes is associated with advanced liver disease. Although noninvasive biomarkers of fibrosis have been developed for hepatitis C, to date, a fully validated, noninvasive biomarker of fibrosis for NAFLD does not exist.
As noted, the spectrum of NAFLD also includes hepatocellular carcinoma, and in a series of 105 patients with hepatocellular carcinoma, hepatitis C virus accounted for 51% and cryptogenic liver disease accounted for another 29%. Since cases of cryptogenic cirrhosis in the United States are considered to be “burned out NASH,” approximately 13% of patients with hepatocellular carcinoma may have had underlying NAFLD as the cause of their liver disease.23 These data suggest that, similar to other cirrhotic patients, NAFLD patients with cirrhosis should be screened for hepatocellular carcinoma.