Atrial fibrillation: New drugs, devices, and procedures
ABSTRACTThe way atrial fibrillation is managed has changed in recent years. Although new anticoagulants are available and more are coming, they offer only marginal benefit over warfarin (Coumadin) and have the disadvantages that their levels cannot be monitored and that their effect cannot be reversed rapidly if bleeding develops. Attempts should be made to control symptomatic atrial fibrillation, first with antiarrhythmic drugs, then with radiofrequency ablation or with a combination. Ablation can be repeated for fibrillation that persists after the first few months.
KEY POINTS
- Warfarin is as safe as—and more effective than—the combination of aspirin and clopidogrel (Plavix) if the international normalized ratio is in the therapeutic range 65% of the time or more.
- New anticoagulants are promising alternatives to warfarin, but they also pose risks. Patients who are doing well on warfarin need not change.
- Several antiarrhythmic drugs are available to control symptomatic atrial fibrillation. Dronedarone (Multaq) should only be considered for patients with paroxysmal atrial fibrillation without significant cardiovascular disease.
- Ablation is often effective in controlling atrial fibrillation, but recurrence is common. Early recurrence often subsides, but late recurrence often requires a repeat procedure.
Clinical trials show that alternatives are marginally better than warfarin
In the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial,8 dabigatran was associated with a significantly lower incidence of intracranial hemorrhage, combined strokes, and systemic embolization than warfarin. The incidence of major bleeds was slightly lower with dabigatran. Although dabigatran performed better, the differences were small and would not require patients to change from warfarin if they are already doing well.
Apixaban and rivaroxaban are other alternatives to warfarin, with different mechanisms of action and metabolism. Although rivaroxaban’s half-life is similar to that of apixaban and dabigatran, it is being marketed as allowing once-daily dosing instead of twice-daily.
Recent randomized controlled clinical trials of the new drugs include:
- The Apixaban Versus Acetylsalicylic Acid (ASA) to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment (AVERROES) trial,9 which compared apixaban and aspirin
- The Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial comparing apixaban and warfarin10
- The Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF),11 comparing rivaroxaban and warfarin
- RE-LY,8 comparing dabigatran and warfarin.
In the ARISTOTLE,10 ROCKET AF,11 and RE-LY trials,8 the time that the warfarin patients’ INRs were in the therapeutic range varied from 55% to 68%. This seems low and is a problem when trying to compare therapies, but is probably about as high as one can expect in the real world.
In AVERROES,9 the combined rate of stroke and embolism was higher with aspirin than with apixaban. In the other trials, the rates were slightly better with the new drugs than with warfarin, and the rates of major hemorrhage and hemorrhagic stroke were only slightly higher with warfarin than with the new drugs. Because the differences in benefits and risks are so small, the main advantage of the newer drugs will probably be for patients who have difficulty staying in the therapeutic INR range on warfarin.
RATE CONTROL VS RESTORATION OF SINUS RHYTHM
Evidence is insufficient to determine the risk of very-long-term asymptomatic atrial fibrillation in patients on appropriate anticoagulation. Rate control is an option for asymptomatic patients but provides no change in quality of life and no definitive reduction in the risk of stroke. The main argument for restoring normal sinus rhythm in patients with mild to moderate symptoms is that it improves exercise capacity. The need for anticoagulation persists when patients are converted to sinus rhythm because the risk of recurrent atrial fibrillation remains high.
For patients with symptomatic atrial fibrillation, rate control is sometimes achieved with beta-blockers or calcium channel blockers. Rate control may be augmented with the addition of digoxin, but when used alone digoxin generally does not control the rate of atrial fibrillation. However, in many cases of atrial fibrillation, symptoms are not rate-related, and cardioversion to normal sinus rhythm should be attempted. In such cases, the symptoms may be attributable to a loss of atrial transport function.
Patients with the following risk factors should be admitted to the hospital to start antiarrhythmic drugs:
- Borderline or a long QTc interval at baseline (> 450 msec)
- Treatment with dofetilide (Tikosyn) because of its effects on the QT interval
- Heart failure or poor left-ventricular function
- Sinus node dysfunction
- Significant atrioventricular conduction disease.
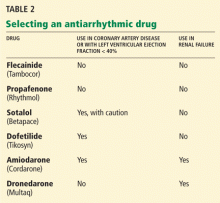
Selecting an antiarrhythmic drug
Any of the antiarrhythmic drugs listed in Table 2 can be used for a patient with lone atrial fibrillation (ie, not caused by underlying heart disease). The choice of drug should be determined by whether coronary artery disease or renal failure is present as well. Liver disease or chronic obstructive pulmonary disease also may affect this decision.
Benefits of dronedarone are mixed
In a randomized trial of dronedarone vs placebo in patients with atrial fibrillation, the rate of death and the rate of first hospitalization due to a cardiovascular event at 21 months were significantly lower with dronedarone.12 No difference was found between the two groups in the rate of death from all causes, but fewer people died of cardiovascular causes in the dronedarone group. More patients taking dronedarone developed bradycardia, QT-interval prolongation, nausea, diarrhea, rash, or a higher serum creatinine level. Gastrointestinal side effects are often a problem with dronedarone: 20% to 30% of patients cannot tolerate the drug.
Dronedarone may cause a small rise in creatinine, and although this effect should be monitored, by itself it should not be interpreted as impairment of renal function. In a study in healthy people,13 dronedarone caused a 10% to 15% increase in serum creatinine, but the glomerular filtration rate was unchanged, as were renal plasma flow and anion secretion.
Another trial, in patients with severe heart failure, found that patients taking dronedarone had higher rates of hospitalization and overall mortality, raising serious concern about the safety of this drug in patients with advanced heart failure.14
Singh et al15 pooled the data from two multicenter, randomized trials that compared dronedarone with placebo for maintaining sinus rhythm in patients with atrial fibrillation or flutter. The mean time to the recurrence of atrial fibrillation was 116 days with dronedarone and 53 days with placebo. Other trials also showed longer times to recurrence and lower recurrence rates with dronedarone. Although the differences were statistically significant, they may not be clinically meaningful for patients.
Dronedarone is structurally similar to amiodarone (Cordarone), but the two drugs work differently. A meta-analysis of clinical trials16 found that amiodarone recipients had a lower rate of recurrence of atrial fibrillation than did those receiving dronedarone.
Two safety warnings for dronedarone
In January 2011, the US Food and Drug Administration (FDA) issued an alert about cases of rare but severe liver injury in patients treated with dronedarone, including two cases of acute liver failure leading to liver transplantation.17
The Permanent Atrial Fibrillation Outcome Study Using Dronedarone on Top of Standard Therapy (PALLAS)18 compared dronedarone and placebo in patients with permanent atrial fibrillation. More people died or had serious cardiovascular adverse events in the dronedarone group. The study was stopped early after data monitoring showed that rates of death, stroke, and hospitalization for heart failure were two times higher in patients receiving dronedarone. This prompted the FDA to issue another safety alert in July 2011.
Interestingly, the PALLAS study did not set out to determine whether dronedarone controls atrial fibrillation, as the study patients had long-standing, persistent atrial fibrillation. The study was designed only to determine if the drug reduces the rate of adverse events; it clearly does not, and the study shows that dronedarone should not be used to control the heart rate in patients with persistent atrial fibrillation. Instead, its use is best restricted to patients with paroxysmal atrial fibrillation without significant cardiovascular disease.