Prevention and treatment of influenza in the primary care office
ABSTRACTInfluenza, a common respiratory infection, is a source of significant rates of illness, death, and loss of productivity. Annual vaccination is safe and effective in preventing disease and in reducing its severity. Yet a majority of eligible US adults do not receive the annual vaccine, at least in part because of misunderstandings about adverse reactions and clinical effectiveness.
KEY POINTS
- Influenza vaccination is effective at preventing influenza-associated disease.
- Influenza vaccine is safe in people with a history of mild egg allergy.
- Many new vaccine formulations exist and may offer benefits to different patient groups.
- Neuraminidase inhibitors are recommended for treatment and postexposure prophylaxis in patients at high risk of influenza-related complications; however, they are not a substitute for vaccination.
Every year, 5% to 20% of US residents contract the flu, 200,000 are hospitalized for it, and 36,000 die of influenza-related complications. The economic impact, including direct medical costs and lost earnings, exceeds $87 billion.1 Despite this, less than half of eligible US residents were vaccinated in the 2012–2013 season, with uninsured people more than twice as likely to forgo vaccination.2,3
Several studies have shown that influenza vaccination reduces the need for outpatient encounters and hospitalizations and lowers the incidence of death from acute myocardial infarction, the rate of all-cause mortality, and even the incidence of therapies administered by implantable defibrillators.4–6 In the 2012–2013 influenza season, vaccination prevented an estimated 3.2 million medically attended illnesses and almost 80,000 hospitalizations; 70% of hospitalizations prevented were in children age 6 months to 4 years and in adults over age 65.7
After the 2009 H1N1 pandemic, which disproportionately killed previously healthy adults, the US Centers for Disease Control and Prevention (CDC) expanded its vaccination recommendations to include everyone above the age of 6 months, with few contraindications.8
In addition, recent years have seen a great expansion in vaccine options, changes in the at-risk demographics, and continued widespread resistance to certain antiviral agents, with implications for practice in primary care.
Here, we review the barriers and the new options for treatment and prevention of influenza.
HEMAGGLUTININ AND NEURAMINIDASE
Influenza infection is caused by one of the circulating strains of influenza virus A or B.
The major viral surface glycoproteins are hemagglutinin and neuraminidase. Hemagglutinin plays an important role in viral attachment to host cells and is the major immunogen in the influenza vaccine. Neuraminidase contains an active enzymatic site that cleaves the newly formed budding influenza viruses from host-cell sialic acid residues and allows them to be released from the cell membrane to infect other respiratory epithelial cells. It is the target of currently recommended antiviral drugs.
VACCINE PRODUCTION
Throughout the year, 130 influenza centers around the world sample circulating strains and share their data with five World Health Organization (WHO) Collaborating Centers for Reference and Research on Influenza. The WHO analyzes the circulation patterns, predicts the strains most likely to be circulating in the next influenza season, and shares these strains with manufacturers of the vaccine.
Pharmaceutical companies then begin an elaborate process of producing and distributing hundreds of millions of doses of vaccine worldwide. The production traditionally uses millions of fertilized chicken eggs to produce strain-specific influenza hemagglutinin. Individual vaccine strains are combined into the final product after being inactivated by chemical or physical splitting of the viral envelope with or without subsequent purification of the hemagglutinin particles.
Before 2013, the WHO’s yearly recommendations included two strains of influenza A and a single strain of influenza B. In 2013, new quadrivalent vaccines that include protection against a second strain of influenza B were approved.
The WHO strain-selection process allows manufacturers about 6 months to produce the vaccine. In a typical year, the worldwide demand is about 400 million doses. The theoretical maximal annual worldwide capacity, given current techniques, is fewer than 1 billion doses, which is well short of the 10 billion doses necessary to allow for the double vaccination needed in a pandemic.9 Newly approved recombinant manufacturing techniques offer greater production efficiency, while novel methods of intradermal administration increase vaccine immunogenicity, decreasing the amount of viral antigens used per dose.
INACTIVATED VS LIVE-ATTENUATED
In addition to intramuscular inactivated influenza vaccine, a live-attenuated vaccine in the form of an intranasal spray (FluMist) became available in 2003. This form is generally favored in children, as it avoids the discomfort of an injection. It contains live, weakened, cold-adapted influenza strains that reproduce in the relatively colder temperatures of the exterior nares but cannot survive in the warmer temperatures of the lung and proximal airways. It is approved for healthy people 2 to 49 years of age, and some evidence suggests that it may be more effective than inactivated influenza vaccine in children,10 although its utility is limited by multiple contraindications (see below).
INFLUENZA VACCINE INDICATIONS AND CONTRAINDICATIONS
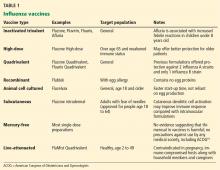
Vaccination for influenza is recommended for all persons 6 months of age and older, an expansion from pre-2009 guidelines that did not recommend vaccination for healthy adults age 19 to 49 who were not in contact with people at high risk of influenza-related complications.8 Many new vaccine formulations have become available in recent years, each with specific benefits, risks, and target populations (Table 1).
Contraindications to inactivated vaccine
The only firm contraindication to inactivated influenza vaccine is previous severe allergic reaction to influenza vaccine or any of its components. Those with moderate to severe acute illness are advised to wait until their condition improves before being vaccinated. People who have had Guillain-Barré syndrome and those with egg allergy are discussed in MISAPPREHENSIONS THAT POSE BARRIERS TO VACCINATION, below. There is no risk of influenza infection from inactivated influenza vaccine.
Contraindications to live-attenuated influenza vaccine
Unlike inactivated influenza vaccine, the live-attenuated vaccine does result in shedding of vaccine-strain virus from the vaccinated host, with the theoretical potential for transmission of the virus from the vaccine recipient to other people, as well as the potential for influenza-like illness in vaccine recipients.11,12 Based on reported events, the former is estimated to occur in 10 to 20 per 1 million vaccinations, although these cases have never been proven to be caused by a cold-adapted vaccine-strain rather than by coincidental transmission of circulating wild-type viral strains.13
Despite this exceedingly small risk of viral transmission, live-attenuated influenza vaccine has multiple contraindications, including age less than 2 years and more than 49 years, disease- or drug-related compromised immune status, pregnancy, egg allergy, and history of allergic reaction to the formulation. These limit its use and are important to review in detail before prescribing.14
Use of neuraminidase inhibitors within 2 days before or 2 weeks after receiving live-attenuated influenza vaccine may interfere with replication of the cold-adapted strain and decrease the vaccine’s effectiveness.14