Type 2 diabetes: Evolving concepts and treatment
ABSTRACT
In view of new information, we are revising the way we think about and treat diabetes mellitus. In this new view, the insulin-producing beta cells are key, and preserving beta-cell function is paramount. These insights, together with recent outcome studies provide compelling arguments regarding treatments of choice.
KEY POINTS
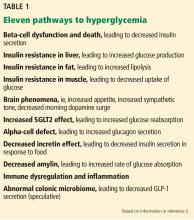
- At least 11 pathways lead to hyperglycemia; of these, beta-cell dysfunction is central.
- As different classes of diabetes drugs act on different pathways, we can target the pathways contributing to hyperglycemia in the individual patient, using fewer agents and lessening the risk of hypoglycemic episodes.
- In selecting treatment, we should favor drugs that are “gentle” on beta cells, do not cause dangerous hypoglycemia, and improve long-term outcomes as shown in randomized clinical trials.
Insights from basic and clinical research are changing the way we treat diabetes mellitus. In 2016, several key diabetes organizations, ie, the American Diabetes Association (ADA), the Juvenile Diabetes Research Foundation (JDRF), the European Association for the Study of Diabetes (EASD), and the American Association of Clinical Endocrinologists (AACE), called for bringing therapeutic approaches in line with our updated understanding of disease pathophysiology, replacing “one-size-fits-all” management with a tailored approach.1 This message has since been reiterated.2
Here, we review advances in our understanding of diabetes and how these inform a new model of diabetes treatment.
BETA CELLS ARE KEY
High levels of glucose and lipids damage and eventually kill beta cells through mechanisms including that of oxidative stress, so that glucose control deteriorates over time. The same processes are active in the target-organ damage seen in diabetes.3,4 These 2 insights—that the disease arises from combinatorial, nondiscrete pressures and that it proceeds through common processes of cell damage—leads us to a more unified understanding of the mechanism of diabetes, and may eventually replace current classifications of type 1, type 2, or latent autoimmune diabetes in adults, as well as nomenclature such as “microvascular” and “macrovascular” disease.3
FIRST-LINE LIFESTYLE INTERVENTIONS
Lifestyle interventions are the first-line therapy for elevated blood glucose. Achieving and maintaining a healthy body mass index is essential to help correct insulin resistance and minimize beta-cell dysfunction.
Lifestyle modifications for overweight or obese patients with diabetes mellitus include optimal caloric intake, decreased intake of simple carbohydrates, increased physical activity, and a 3% to 5% reduction in body weight.5 Weight-loss drugs may be indicated in obese patients. Normalization of lipids and hypertension should be an early goal.
RIGHT MEDICATIONS, RIGHT PATIENTS
While all of the drugs approved for treating diabetes lower glucose levels, some are more beneficial than others, possessing actions beyond their effect on plasma glucose levels, both good and bad.
The AACE guideline for use of various antidiabetic medications6 grades factors such as risks of hypoglycemia, ketoacidosis, weight gain, cardiovascular events, and renal, gastrointestinal, and bone concerns. This represents a much-needed first step toward guidance on selecting the right medications for the right patients. Risk factors (such as heart failure) and comorbidities (such as nonalcoholic fatty liver disease and nonalcoholic steatohepatitis) are among the considerations for choosing treatment.
Two principles
We propose 2 principles when choosing treatment:
Use “gentle” agents, ie, those that are least likely to exhaust beta cells or damage the organs involved in diabetes-related complications. Since the disease course depends on the health of the beta cells, give preference to agents that appear to best support beta cells—ie, agents that create the least oxidative stress or wear-and-tear—as will be outlined in this article.
Diabetes is associated with risks of cardiovascular disease, cardiac events, heart failure, and accelerated renal decompensation. Thus, it is equally important to prevent damage to the cardiovascular system, kidneys, and other tissues subject to damage through glucolipotoxicity.
Balancing glycemic control and risk
The hemoglobin A1c level is the chief target of care and an important barometer of risk of diabetes-related complications. In 2018, the American College of Physicians (ACP) relaxed its target for hemoglobin A1c from 7% to 8%.8 This move was apparently to give physicians greater “wiggle room” for achieving goals in hypoglycemia-prone patients. This, however, may take a toll.
Hypoglycemia is closely tied to cardiovascular disease. Even mild and asymptomatic hypoglycemia that goes undiagnosed and unnoticed by patients has been found to be associated with higher rates of all-cause mortality, prolonged QT interval, angina, arrhythmias, myocardial dysfunction, disturbances in autonomic balance, and sudden death.9–11
However, the ADA, AACE, American Association of Diabetes Educators (AADE), and the Endocrine Society jointly issued a strong indictment of the ACP recommendation.12 They argue that tight glucose control and its well-documented “legacy effects” on long-term outcomes should not be sacrificed.12,13 Indeed, there is no need to abandon evidence-based best practices in care when at least 8 of the 11 classes of antidiabetes agents do not introduce the same level of risk for hypoglycemia.
Current guidelines argue for tight glucose control but generally stop short of discriminating or stratifying the mechanisms of action of the individual classes of drugs. These guidelines also do not stress targeting the particular pathways of hyperglycemia present in any given patient. However, the 2016 ADA joint statement acknowledges the need to “characterize the many paths to beta-cell dysfunction or demise and identify therapeutic approaches that best target each path.”1