An obese 48-year-old man with progressive fatigue and decreased libido
Release date: May 1, 2019
Expiration date: April 30, 2020
Estimated time of completion: 1 hour
Click here to start this CME/MOC activity.
CASE CONTINUED: BEGINNING TREATMENT
The physician counsels the patient regarding the implications, potential adverse outcomes, and available treatments for his obesity, including lifestyle modification and bariatric surgery. The patient declines surgery and wishes to adopt a weight-reducing diet and exercise program, for which he is referred to a dietitian.
In addition, in view of the patient’s clinically and biochemically proven hypogonadism, his physician offers testosterone replacement therapy. He orders a serum prostate-specific antigen (PSA) level, which is 1.3 ng/dL (reference range < 4 ng/dL). The patient is prescribed 5 g of 1% testosterone gel daily.
TESTOSTERONE REPLACEMENT THERAPY
4. Which is the most common adverse effect of testosterone replacement therapy?
- Cardiovascular events
- Erythrocytosis
- Prostate cancer
- Infertility
- Obstructive sleep apnea
Clinicians should be very cautious in initiating testosterone replacement therapy in any patient with an unstable medical condition.
There are several formulations of testosterone replacement therapy, including intramuscular injections, transdermal gels or patches, buccal tablets, an intranasal gel, and oral tablets. Of note, there are 2 different forms of oral testosterone preparations: testosterone undecanoate and 17-alpha alkylated testosterone. The former is unavailable in the United States and the latter is not recommended for use due to its proven hepatic toxicity.41
Testosterone and erythrocytosis
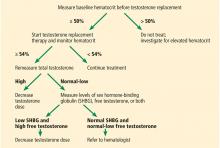
Meta-analyses have concluded that the most frequent adverse event of testosterone replacement therapy is a significant rise in hematocrit.42 This rise was found to be dose-dependent and was more marked in older men.43 Although all preparations can cause erythrocytosis, parenteral forms have been observed to raise it the most, particularly short-term injectables.44,45
The mechanism behind this increase is attributed to increased erythropoietin levels and improved usage of iron for red blood cell synthesis.46 In fact, testosterone replacement therapy has been shown to improve hemoglobin levels in patients with anemia.47 On the other hand, increasing hematocrit levels may lead to thrombotic and vasoocclusive events.44
Testosterone and prostate cancer
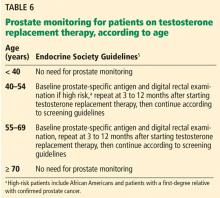
The relationship between testosterone treatment and prostate cancer has long been studied. Historically, testosterone replacement therapy was believed to increase the risk of prostate cancer; however, recent studies and meta-analyses have shown that this is not the case.42,48 Nevertheless, clinical guidelines still recommend prostate monitoring for men on testosterone replacement therapy.5,6
Testosterone and cardiovascular risk
The evidence regarding this issue has been contradictory and inconsistent. Meta-analyses have demonstrated that low testosterone is associated with higher risk of major adverse cardiovascular events.50 These studies argue for the use of testosterone replacement therapy in hypogonadal men to decrease the risk. However, other studies and meta-analyses have found that testosterone replacement therapy is associated with increased cardiovascular risk and have concluded that major adverse cardiac events are in fact a risk of testosterone replacement therapy.51
Current recommendations advocate against the use of testosterone replacement therapy in men with uncontrolled heart failure or with cardiovascular events in the past 3 to 6 months.5,6 Cardiovascular risk factors should be addressed and corrected, and patients should be educated on cardiovascular symptoms and the need to report them if they occur.
Testosterone and infertility
As described earlier, testosterone replacement therapy increases negative feedback on the pituitary and decreases LH and FSH production, leading to less spermatogenesis. Other treatment options should be sought for hypogonadal men wishing to preserve fertility.
Other adverse effects
Other adverse effects of testosterone replacement therapy include acne, oily skin, obstructive sleep apnea, gynecomastia, and balding.
Given all the adverse events that can be associated with testosterone replacement therapy, the risks and benefits of treating hypogonadism in each patient should be taken into consideration, and an individualized approach is required.