Influenza update 2018–2019: 100 years after the great pandemic
ABSTRACT
Four influenza pandemics, starting with the historic 1918 pandemic, have killed thousands of people around the world. Vaccination, still the most important means of preventing influenza, is currently recommended yearly for all people age 6 months and older, with a goal of vaccinating 80% of all Americans and 90% of at-risk populations. Neuraminidase inhibitors are underused, and a new drug with a different mechanism of action, baloxavir marboxil, is expected to be approved soon in the United States.
KEY POINTS
- Influenza A(H7N9) is a prime candidate to cause the next influenza pandemic.
- Influenza vaccine prevents 300 to 4,000 deaths in the United States every year.
- The 2018–2019 quadrivalent influenza vaccine contains updated A(H3N2) and B/Victoria lineage components different from those in the 2017–2018 Northern Hemisphere vaccine.
- The live-attenuated influenza vaccine, which was not recommended during the 2016–2017 and 2017–2018 influenza seasons, is recommended for the 2018–2019 influenza season.
- Influenza vaccine is recommended any time during pregnancy and is associated with lower infant mortality rates.
- Overall influenza vaccination rates remain below the 80% target for all Americans and 90% for at-risk populations.
VACCINATION IN SPECIAL POPULATIONS
High-dose vaccine for older adults
The high-dose influenza vaccine has been licensed since 2009 for use in the United States for people ages 65 and older.
Recent studies confirmed that high-dose vaccine is more effective than standard-dose vaccine in veterans34 and US Medicare beneficiaries.35
The high-dose vaccine is rapidly becoming the primary vaccine given to people ages 65 and older in retail pharmacies, where vaccination begins earlier in the season than in providers’ offices.36 Some studies have shown that the standard-dose vaccine wanes in effectiveness toward the end of the influenza season (particularly if the season is long) if it is given very early. It remains to be seen whether the same applies to the high-dose influenza vaccine.
,Some advocate twice-annual influenza vaccination, particularly for older adults living in tropical and subtropical areas, where influenza seasons are more prolonged. However, a recently published study observed reductions in influenza-specific hemagglutination inhibition and cell-mediated immunity after twice-annual vaccination.37
Vaccination is beneficial during pregnancy
Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants.
One recently published study showed that 18% of infants who developed influenza required hospitalization.38 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively.
Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.39
Some studies have shown that influenza virus infection can increase susceptibility to certain bacterial infections. A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.40
Factors that make vaccination less effective
Several factors including age-related frailty and iatrogenic and disease-related immunosuppression can affect vaccine effectiveness.
Frailty. A recent study showed that vaccine effectiveness was 77.6% in nonfrail older adults but only 58.7% in frail older adults.41
Immunosuppression. Temporary discontinuation of methotrexate for 2 weeks after influenza vaccination in patients with rheumatoid arthritis improves vaccine immunogenicity without precipitating disease flare.42 Solid-organ and hematopoietic stem cell transplant recipients who received influenza vaccine were less likely to develop pneumonia and require intensive care unit admission.43
The high-dose influenza vaccine is more immunogenic than the standard-dose vaccine in solid-organ transplant recipients.44
Statins are widely prescribed and have recently been associated with reduced influenza vaccine effectiveness against medically attended acute respiratory illness, but their benefits in preventing cardiovascular events outweigh this risk.45
FUTURE VACCINE CONSIDERATIONS
Moving away from eggs
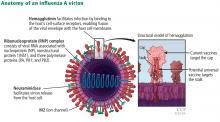
During the annual egg-based production process, which takes several months, the influenza vaccine acquires antigenic changes that allow replication in eggs, particularly in the hemagglutinin protein, which mediates receptor binding. This process of egg adaptation may cause antigenic changes that decrease vaccine effectiveness against circulating viruses.
The cell-based baculovirus influenza vaccine grown in dog kidney cells has higher antigenic content and is not subject to the limitations of egg-based vaccine, although it still requires annual updates. A recombinant influenza vaccine reduces the probability of influenza-like illness by 30% compared with the egg-based influenza vaccine, but also still requires annual updates.46 The market share of these non-egg-based vaccines is small, and thus their effectiveness has yet to be demonstrated.
The US Department of Defense administered the cell-based influenza vaccine to about one-third of Armed Forces personnel, their families, and retirees in the 2017–2018 influenza seasons, and data on its effectiveness are expected in the near future.47
A universal vaccine would be ideal
The quest continues for a universal influenza vaccine, one that remains protective for several years and does not require annual updates.48 Such a vaccine would protect against seasonal epidemic influenza drift variants and pandemic strains. More people could likely be persuaded to be vaccinated once rather than every year.
An ideal universal vaccine would be suitable for all age groups, at least 75% effective against symptomatic influenza virus infection, protective against all influenza A viruses (influenza A, not B, causes pandemics and seasonal epidemics), and durable through multiple influenza seasons.51
Research and production of such a vaccine are expected to require funding of about $1 billion over the next 5 years.
Boosting effectiveness
Estimates of influenza vaccine effectiveness range from 40% to 60% in years when the vaccine viruses closely match the circulating viruses, and variably lower when they do not match. The efficacy of most other vaccines given to prevent other infections is much higher.
New technologies to improve influenza vaccine effectiveness are needed, particularly for influenza A(H3N2) viruses, which are rapidly evolving and are highly susceptible to egg-adaptive mutations in the manufacturing process.
In one study, a nanoparticle vaccine formulated with a saponin-based adjuvant induced hemagglutination inhibition responses that were even greater than those induced by the high-dose vaccine.52
Immunoglobulin A (IgA) may be a more effective vaccine target than traditional influenza vaccines that target IgG, since different parts of IgA may engage the influenza virus simultaneously.53
Vaccines can be developed more quickly than in the past. The timeline from viral sequencing to human studies with deoxyribonucleic acid plasmid vaccines decreased from 20 months in 2003 for the severe acquired respiratory syndrome coronavirus to 11 months in 2006 for influenza A/Indonesia/2006 (H5), to 4 months in 2009 for influenza A/California/2009 (H1), to 3.5 months in 2016 for Zika virus.54 This is because it is possible today to sequence a virus and insert the genetic material into a vaccine platform without ever having to grow the virus.