Genitourinary syndrome of menopause in breast cancer survivors: Treatments are available
ABSTRACT
When treating the genitourinary syndrome of menopause (GSM) in women with breast cancer or at high risk of breast cancer, clinicians must balance the higher cancer risks associated with hormonal treatments against the severity of GSM symptoms, which can be exacerbated by breast cancer treatments. Options for patients who need hormonal therapy include locally applied estrogens, dehydroepiandrosterone (DHEA), and estrogen receptor agonists/antagonists, which vary in their impact on breast cancer risk.
KEY POINTS
- In general, locally applied hormonal therapies relieve GSM symptoms without increasing breast cancer risk.
- DHEA relieves vaginal symptoms without increasing serum estrogen levels.
- Ospemifene has antiestrogenic effects on breast tissue that make it an attractive option for women with breast cancer.
- The combination of conjugated estrogens and bazedoxifene offers a progesterone-free treatment for GSM symptoms in women desiring systemic hormone therapy.
Many breast cancer survivors and women at high risk of breast cancer suffer from genitourinary syndrome of menopause (GSM), a term that encompasses any urinary, genital, or sexual dysfunction related to a hypoestrogenic state. Although GSM is usually caused by postmenopausal estrogen loss, it can also be caused by cancer treatments such as chemotherapy, radiation, and systemic endocrine therapy (eg, tamoxifen, aromatase inhibitors). These treatments can substantially decrease systemic estrogen levels, causing GSM symptoms that can profoundly worsen quality of life.
Managing GSM in these women poses a dilemma because systemic estrogen-containing therapies can increase the risk of breast cancer, and nonhormonal vaginal lubricants and moisturizers provide only minimal benefit. Fortunately, there are hormonal options, including locally applied estrogen, intravaginal dehydroepiandrosterone (DHEA), and estrogen receptor agonists/antagonists (ospemifene and bazedoxifene).
Here, we review the clinical management of GSM in breast cancer survivors and women at high risk of breast cancer and the efficacy and safety of available treatments, including their impact on breast cancer risk.
DRYNESS, IRRITATION, ATROPHY
The term GSM describes vulvovaginal and genitourinary symptoms associated with estrogen loss after menopause. Common symptoms are vaginal dryness, dyspareunia, irritation of genital skin, and pruritus.
LOCAL ESTROGEN THERAPY
Systemic estrogen therapy is widely used and effective for GSM, but there are concerns that it could increase the risk of breast cancer. After the Women’s Health Initiative in 2002 showed higher rates of cardiovascular disease and breast cancer with systemic estrogen-progestin use,5 the use of this hormone therapy declined by approximately 80%.6 Since then, healthcare providers have turned to local (ie, vaginal) estrogen therapies to manage GSM. These therapies have several advantages over systemic hormone therapy:
- Lower risk of adverse effects on the breast and cardiovascular system
- Greater efficacy in treating GSM
- In general, no need for progesterone when low-dose local estrogen is given to a woman with a uterus.7
Is locally applied estrogen systemically absorbed?
Despite these advantages, concerns remain as to whether vaginal estrogen therapy has adverse consequences associated with systemic absorption, particularly from atrophic vaginal tissues.
Santen,8 in a 2015 review of 33 studies, concluded that systemic absorption from low-dose vaginal estrogen is minimal, which provides some rationale for using it to treat vulvovaginal atrophy in postmenopausal women. This finding also suggests that the US Food and Drug Administration (FDA) “black box” warning of possible toxicities with vaginal estrogen is likely overstated, given that serum estrogen levels remained within normal postmenopausal levels.
Nevertheless, many providers are apprehensive about prescribing vaginal estrogen in women with a history of breast cancer because the threshold for systemic estrogen levels associated with breast cancer recurrence has not been established.
ACOG statement. In 2016, a committee of the American College of Obstetricians and Gynecologists cited data showing that low-dose vaginal estrogens do not result in sustained serum estrogen levels exceeding the normal postmenopausal range, and that the use of vaginal estrogens does not increase the risk of cancer recurrence.9 However, they recommend caution with vaginal estrogen use, especially in women with a history of estrogen-dependent breast cancer, reserving it for patients with GSM symptoms nonresponsive to nonhormonal treatment and specifying that it be used in low doses.
Vaginal estrogen formulations
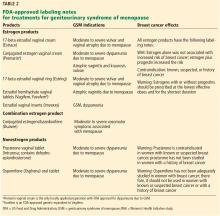
Several types of locally applied estrogens are available, each with different properties and affinity for estrogen receptors. These include conjugated estrogens, 17-beta estradiol, estradiol acetate, and estradiol hemihydrate. Three delivery systems are FDA-approved: creams, rings, and tablets (Table 2).
Vaginal creams. Two vaginal creams are available, one (Estrace) containing 17-beta estradiol and the other (Premarin) containing conjugated estrogens.
The FDA-approved dosage for 17-beta estradiol is 2 to 4 g/day for 1 or 2 weeks, then gradually reduced to half the dose for a similar time. Maintenance dosing is 1 g 1 to 3 times per week. However, the ACOG statement notes that the FDA-approved dosages are higher than those proven to be effective and currently used in clinical practice, eg, 0.5 g twice a week.9
The FDA-approved dosage of conjugated estrogen cream for moderate to severe dyspareunia is 0.5 g daily for 21 days, then off for 7 days, or 0.5 g twice a week.
Vaginal tablets. The vaginal tablet Vagifem and its generic equivalent Yuvafem contain 10 µg of estradiol hemihydrate. The FDA-approved dosage is 10 µg daily for 2 weeks, followed by 10 µg twice a week, inserted into the lower third of the vagina. This dosage is significantly lower than that of estrogen creams.
Vaginal insert. A newly approved vaginal insert (Imvexxy) contains estradiol 4 µg (the lowest dose of vaginal estradiol available) or 10 µg, in a coconut oil vehicle. Its indications are for moderate to severe dyspareunia due to menopause and atrophic vaginitis due to menopause. A study cited in its package insert (www.accessdata.fda.gov/drugsatfda_docs/label/2018/208564s000lbl.pdf) showed that, in patients who used this product, systemic absorption of estradiol remained within the postmenopausal range. Its effects on breast cancer have not yet been studied.
Vaginal rings. Two vaginal rings are marketed. One (Estring) contains 17-beta estradiol, and the other (Femring) contains estradiol acetate. Only the 17-beta estradiol ring delivers a low dose to vaginal tissues, releasing 7.5 µg/day for 90 days. The estradiol acetate ring releases 0.05 mg/day or 0.10 mg/day and is a systemic treatment meant to be used with a progestin, not for local therapy.