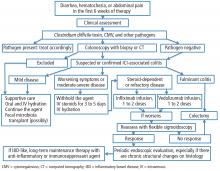
How should we diagnose and manage checkpoint inhibitor-associated colitis?
If a patient experiences diarrhea, hematochezia, or abdominal pain within the first 6 weeks of therapy with one of the anticancer drugs known as immune checkpoint inhibitors (ICIs), the first step is to rule out infection, especially with Clostridium difficile. The next step is colonosocopy with biopsy or computed tomography.
Patients with mild ICI-associated colitis may need only supportive care, and the ICI can be continued. In moderate or severe cases, the agent may need to be stopped and corticosteroids and other colitis-targeted agents may be needed. Figure 1 shows our algorithm for diagnosing and treating ICI-associated colitis.
POWERFUL ANTICANCER DRUGS
ICIs are monoclonal antibodies used in treating metastatic melanoma, non-small-cell lung cancer, metastatic prostate cancer, Hodgkin lymphoma, renal cell carcinoma, and other advanced malignancies.1,2 They act by binding to and blocking proteins on T cells, antigen-presenting cells, and tumor cells that keep immune responses in check and prevent T cells from killing cancer cells.1 For example:
- Ipilimumab blocks cytotoxic T lymphocyte-associated antigen 4
- Nivolumab and pembrolizumab block programmed cell death protein 1
- Atezolizumab blocks programmed death ligand 1.1
With these proteins blocked, T cells can do their job, often producing dramatic regression of cancer. However, ICIs can cause a range of immune-related adverse effects, including endocrine and cutaneous toxicities, iridocyclitis, lymphadenopathy, neuropathy, nephritis, immune-mediated pneumonitis, pancreatitis, hepatitis, and colitis.3
ICI-ASSOCIATED COLITIS IS COMMON
ICI-associated colitis is common; it is estimated to affect about 30% of patients receiving ipilimumab, for example.4 Clinical presentations range from watery bowel movements, blood or mucus in the stool, abdominal cramping, and flatulence to ileus, colectasia, intestinal perforation, and even death.5
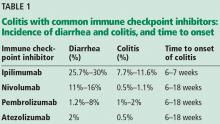
The incidence appears to increase with the dosage and duration of ICI therapy. The onset of colitis typically occurs 6 to 7 weeks after starting ipilimumab,6 and 6 to 18 weeks after starting nivolumab or pembrolizumab.7 Table 1 lists the incidence of diarrhea and colitis and time of onset to colitis with common ICIs. However, colitis, like other immune-related adverse events, can occur at any point, even after ICI therapy has been discontinued.8
It is best to detect side effects of ICIs promptly, as acute inflammation can progress to chronic inflammation within 1 month of onset.9 We believe that early intervention and close monitoring may prevent complications and the need for long-term immunosuppressive treatment.
Patients, family members, and caregivers should be informed of possible gastrointestinal along with systemic side effects. Severe gastrointestinal symptoms such as increased stool frequency and change in stool consistency should trigger appropriate investigation and the withholding of ICI therapy.
COLITIS IS A SPECTRUM
The colon appears to be the gastrointestinal organ most affected by ICIs. Of patients with intestinal side effects, including diarrhea, only some develop colitis. The severity of ICI-associated colitis ranges from mild bowel illness to fulminant colitis.
Hodi et al,10 in a randomized trial in which 511 patients with melanoma received ipilimumab, reported that approximately 30% had mild diarrhea, while fewer than 10% had severe diarrhea, fever, ileus, or peritoneal signs. Five patients (1%) developed intestinal perforation, 4 (0.8%) died of complications, and 26 (5%) required hospitalization for severe enterocolitis.
The pathophysiology of ICI-mediated colitis is unclear. Most cases are diagnosed clinically.
Colitis is graded based on the Montreal classification system11:
Mild colitis is defined as passage of fewer than 4 stools per day (with or without blood) over baseline and absence of any systemic illness.
Moderate is passage of more than 4 stools per day but with minimal signs of systemic toxicity.
Severe is defined as passage of at least 6 stools per day, heart rate at least 90 beats per minute, temperature at least 37.5°C (99.5°F), hemoglobin less than 10.5 g/dL, and erythrocyte sedimentation rate at least 30 mm/h.11
RULE OUT INFECTION
If symptoms such as diarrhea or abdominal pain arise within 6 weeks of starting ICI therapy, then we should check for an infectious cause. The differential diagnosis of suspected ICI-associated colitis includes infections with C difficile, cytomegalovirus, opportunistic organisms, and other bacteria and viruses. ICI-induced celiac disease and immune hyperthyroidism should also be ruled out.4