Diabetes and pregnancy: Risks and opportunities
ABSTRACT
Diabetes in pregnancy increases the risk of adverse maternal, obstetric, fetal, and neonatal outcomes. Internists can reduce these risks by optimizing glycemic control before conception and providing effective counseling on strategies to reduce the risks associated with pregnancy and diabetes. Routine screening of reproductive-age women with diabetes should include a comprehensive physical examination and laboratory tests to identify at-risk patients and begin strategic management. A review of medications for teratogenic potential is also needed.
KEY POINTS
- Aim for a hemoglobin A1c of 6.5% or lower, if it is attainable without increasing the risk of hypoglycemia.
- Avoid teratogenic drugs in sexually active women of childbearing age unless the patient uses effective contraception.
- Because about half of pregnancies are unplanned, it is important to routinely discuss family planning and provide preconception counseling that includes reducing risks associated with pregnancy.
- Screen for diabetic end-organ damage, especially retinopathy and nephropathy.
LABORATORY TESTS TO CONSIDER
Hemoglobin A1c. The general consensus is to achieve the lowest hemoglobin A1c level possible that does not increase the risk of hypoglycemia. The American Diabetes Association (ADA) recommends that, before attempting to conceive, women should lower their hemoglobin A1c to below 6.5%.1
Thyroid measures. Autoimmune thyroid disease is the most common autoimmune disorder associated with diabetes and has been reported in 35% to 40% of women with type 1 diabetes.23 Recommendations are to check thyroid-stimulating hormone and thyroid peroxidase antibody levels before conception or early in pregnancy in all women with diabetes.1,24 Overt hypothyroidism should be treated before conception, given that early fetal brain development depends on maternal thyroxine.
Renal function testing. Preconception assessment of renal function is important for counseling and risk stratification. This assessment should include serum creatinine level, estimated glomerular filtration rate, and urinary albumin excretion.21
Celiac screening. Because women with type 1 diabetes are more susceptible to autoimmune diseases, they should be screened for celiac disease before conception, with testing for immunoglobulin A (IgA) and tissue transglutaminase antibodies, with or without IgA endomysial antibodies.16,25,26 An estimated 6% of patients with type 1 diabetes have celiac disease vs 1% of the general population.25 Celiac disease is 2 to 3 times more common in women, and asymptomatic people with type 1 diabetes are considered at increased risk for celiac disease.26
The association between type 1 diabetes and celiac disease most likely relates to the overlap in human leukocyte antigens of the diseases. There is no established link between type 2 diabetes and celiac disease.25
Undiagnosed celiac disease increases a woman’s risk of obstetric complications such as preterm birth, low birth weight, and stillbirth.26 The most likely explanation for these adverse effects is nutrient malabsorption, which is characteristic of celiac disease. Adherence to a gluten-free diet before and during gestation may reduce the risk of preterm delivery by as much as 20%.26
Vitamin B12 level. Celiac disease interferes with the absorption of vitamin B12-instrinsic factor in the ileum, which can lead to vitamin B12 deficiency. Therefore, baseline vitamin B12 levels should be checked before conception in women with celiac disease. Levels should also be checked in women taking metformin, which also decreases vitamin B12 absorption. Of note, increased folate levels due to taking supplements can potentially mask vitamin B12 deficiency.
MEDICATIONS TO REVIEW FOR PREGNANCY INTERACTIONS
Diabetic medications
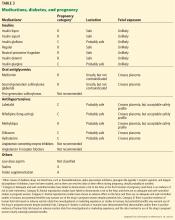
Insulin is the first-line pharmacotherapy for pregnant patients with type 1, type 2, or gestational diabetes. Insulin does not cross the placenta to a measurable extent, and most insulin preparations have been classified as category B,1 meaning no risks to the fetus have been found in humans.
Insulin dosing during pregnancy is not static. Beginning around mid-gestation, insulin requirements increase,28,29 but after 32 weeks the need may decrease. These changes require practitioners to closely monitor blood glucose throughout pregnancy.
Both basal-bolus injections and continuous subcutaneous infusion are reasonable options during pregnancy.30 However, the need for multiple and potentially painful insulin injections daily can lead to poor compliance. This inconvenience has led to studies using oral hypoglycemic medications instead of insulin for patients with gestational and type 2 diabetes.
Metformin is an oral biguanide that decreases hepatic gluconeogenesis and intestinal glucose absorption while peripherally increasing glucose utilization and uptake. Metformin does not pose a risk of hypoglycemia because its mechanism of action does not involve increased insulin production.7
Metformin does cross the placenta, resulting in umbilical cord blood levels higher than maternal levels. Nevertheless, studies support the efficacy and short-term safety of metformin use during a pregnancy complicated by gestational or type 2 diabetes.7,31 Moreover, metformin has been associated with a lower risk of neonatal hypoglycemia and maternal weight gain than insulin.32 However, this agent should be used with caution, as long-term data are not yet available, and it may slightly increase the risk of premature delivery.
Glyburide is another oral hypoglycemic medication that has been used during pregnancy. This second-generation sulfonylurea enhances the release of insulin from the pancreas by binding beta islet cell ATP-calcium channel receptors. Compared with other sulfonylureas, glyburide has the lowest rate of maternal-to-fetal transfer, with umbilical cord plasma concentrations 70% of maternal levels.33 Although some trials support the efficacy and short-term safety of glyburide treatment for gestational diabetes,34 recent studies have associated glyburide use during pregnancy with a higher rate of neonatal hypoglycemia, neonatal respiratory distress, macrosomia, and neonatal intensive care unit admissions than insulin and metformin.1,35
Patients treated with oral agents should be informed that these drugs cross the placenta, and that although no adverse effects on the fetus have been demonstrated, long-term safety data are lacking. In addition, oral agents are ineffective in type 1 diabetes and may be insufficient to overcome the insulin resistance in type 2 diabetes.
Antihypertensive drugs
All antihypertensive drugs cross the placenta, but several have an acceptable safety profile in pregnancy, including methyldopa, labetalol, clonidine, prazosin, and nifedipine. Hydralazine and labetalol are short-acting, come in intravenous formulations, and can be used for urgent blood pressure control during pregnancy. Diltiazem may be used for heart rate control during pregnancy, and it has been shown to lower blood pressure and proteinuria in pregnant patients with underlying renal disease.36,37 The ADA recommends against chronic use of diuretics during pregnancy because of potential reductions in maternal plasma volume and uteroplacental perfusion.1
Angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), and direct renin inhibitors are contraindicated during pregnancy because of the risk of fetal defects, particularly in the renal system.21,38 Although there is evidence to question the association between first semester exposure and fetotoxicity,39 we avoid these drugs during pregnancy and switch to a different agent in women planning pregnancy.
Other drugs
Statins are contraindicated in pregnancy because they interfere with the development of the fetal nervous system.21 Although preliminary data from a small study did not identify safety risks associated with pravastatin use after 12 weeks of gestation,40 we recommend discontinuing statins in women attempting pregnancy.
Aspirin. The US Preventive Services Task Force41 recommends low-dose aspirin (81 mg/day) after 12 weeks of gestation for women with type 1 or type 2 diabetes, as well as those with renal disease or chronic hypertension, to prevent preeclampsia. Of note, higher doses need to be used with caution during pregnancy because fetal abnormalities have been reported, such as disruption of fetal vasculature (mesenteric vessels), gastroschisis, and small intestinal atresia.16
Folate supplementation (0.6–4 mg/day) is recommended in women with celiac disease to prevent neural tube defects in the offspring, and the US Preventive Services Task Force recommends 0.4 mg daily of folic acid supplementation for all women planning or capable of pregnancy.42–44 Higher doses, ranging from 0.6 to 5 mg/day, have been proposed for patients with diabetes,13 and we recommend at least 1 mg for this group, based on data suggesting that higher doses further reduce the risk of neural tube defects.43