The experts debate: Perioperative beta-blockade for noncardiac surgery—proven safe or not?
ABSTRACT
Guidelines on perioperative management of patients undergoing noncardiac surgery recommend the use of prophylactic perioperative beta-blockers in high-risk patients who are not already taking them, and their continuance in patients on chronic beta-blockade prior to surgery. These recommendations were challenged recently by results of the Perioperative Ischemic Evaluation (POISE), a large randomized trial of extended-release metoprolol succinate started immediately before noncardiac surgery in patients at high risk for atherosclerotic disease. While metoprolol significantly reduced myocardial infarctions relative to placebo in POISE, it also was associated with significant excesses of both stroke and mortality. The merits and limitations of POISE and its applicability in light of other trials of perioperative beta-blockade are debated here by two experts in the field—Dr. Don Poldermans and Dr. P.J. Devereaux (co-principal investigator of POISE).
NOTE: The individual co-authors in this debate-based article are responsible only for those views within their respective bylined subsections and those views ascribed to them in the rebuttals and discussion at the end.
Perioperative beta-blockade improves outcomes
By Don Poldermans, MD, PhD
It is my contention that perioperative beta-blockade improves mortality and cardiac outcomes in select high- and intermediate-risk patients undergoing noncardiac surgery. Patients on chronic beta-blocker therapy should have it continued perioperatively. For patients not already on beta-blockade who are at cardiac risk, initiation of low-dose beta-blocker therapy should be considered prior to surgery; such therapy should be started approximately 1 month before surgery, with dose titration to achieve hemodynamic stability. Reports of increased stroke rates with perioperative beta-blockade appear to be due to inappropriate acute administration of high-dose beta-blocker therapy.
THE PHYSIOLOGIC RATIONALE FOR PERIOPERATIVE BETA-BLOCKADE
Perioperative myocardial infarction (MI) can occur by one of two mechanisms, both of which can be attenuated by beta-blockade:
- The stress induced by surgery can cause an asymptomatic coronary plaque to become unstable and rupture, resulting in complete occlusion of a portion of the coronary tree. This type of perioperative MI occurs typically in patients with multiple risk factors for MI absent a critical coronary stenosis. The perioperative risk associated with unstable plaque can be reduced pharmacologically with aspirin, statins, and chronic beta-blocker therapy.
- Alternately, a fixed coronary stenosis can predispose to a mismatch of oxygen demand and supply, leading to myocardial ischemia and infarction. The patient with a fixed coronary lesion typically presents with stable angina pectoris, and the at-risk stenosis is identified through a stress echocardiogram or nuclear scan. The risk conferred by flow-limiting stable plaque can be reduced by coronary revascularization and a short course of beta-blocker therapy prior to surgery.
INITIAL SUPPORTIVE DATA
Mangano and colleagues were the first to evaluate perioperative beta-blockade in a randomized, controlled fashion.1,2 In their study of 200 surgical patients with or at risk for coronary artery disease, oral atenolol administered perioperatively was associated with a 50% reduction (compared with placebo) in the incidence of postoperative myocardial ischemia as measured by three-lead Holter monitoring.2 During 2 years of follow-up, mortality was significantly lower in the atenolol group (10%) than in the placebo group (21%) (P = .019).1
In the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE I), my research group randomized 112 high-risk patients (as identified by dobutamine echocardiography) to standard perioperative care alone or standard perioperative care plus bisoprolol starting 30 days prior to major vascular surgery.3 The dosage of bisoprolol was titrated to achieve a target heart rate of 60 to 70 beats per minute. Thirty days after surgery, the incidence of the primary end point—a composite of death from cardiac causes or nonfatal MI—was reduced from 34% in the standard-care group to 3.4% in the bisoprolol group (P < .001). Thus, in this unblinded study in a population with proven coronary artery disease, beta-blockade clearly improved outcomes.
Additional studies of perioperative beta-blocker use have produced a wide range of outcomes, with most favoring beta-blockade, albeit usually not to a statistically significant degree.4–13 Notably, only some of these trials were randomized, they used various beta-blocker regimens at various doses, they were conducted in patients with varying degrees of cardiac risk, and many had small sample sizes.
What emerged from these trials was the idea that perioperative beta-blockade in patients with coronary artery disease produces an effect similar to that of long-term beta-blockade in reducing the risk of cardiovascular events in post-MI patients and in those with coronary artery disease and heart failure.
THE POISE STUDY AND ITS IMPLICATIONS
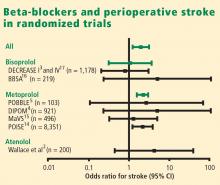
Results of the Perioperative Ischemic Evaluation (POISE) were published in 2008, in which 8,351 noncardiac surgery patients with or at risk of atherosclerotic disease were randomized to placebo or extended-release metoprolol succinate started 2 to 4 hours preoperatively and continued for 30 days.14 Metoprolol was associated with a clear reduction in the primary end point, a composite of cardiovascular death, nonfatal MI, or nonfatal cardiac arrest (5.8% vs 6.9% with placebo; hazard ratio [HR] = 0.84 [95% CI, 0.70–0.99]; P = .0399), but this effect was offset by significant increases in total mortality and stroke incidence in the metoprolol group. Mortality was 3.1% with metoprolol versus 2.3% with placebo (HR = 1.33 [95% CI, 1.03–1.74]; P = .0317), and stroke incidence was 1.0% with metoprolol versus 0.5% with placebo (HR = 2.17 [95% CI, 1.26–3.74]; P = .0053). Cerebral infarction, not bleeding, explained most of the excess mortality with metoprolol.
Of the 60 strokes in POISE, 49 were ischemic in origin, 3 were hemorrhagic, and 8 were of uncertain etiology. Preoperative predictors of stroke were the use of clopidogrel and a history of stroke or transient ischemic attack. Postoperative predictors of stroke included intraoperative bleeding and intraoperative hypotension. These predictors suggest a diseased cerebrovascular tree or unstable hemodynamics during the intraoperative period in the patients who suffered a stroke.
Does dosing explain the rise in mortality and strokes?
Could the fatal outcomes associated with the beta-blocker in POISE be attributed to the dosage of metoprolol? In the study, 100 mg of metoprolol was started immediately prior to surgery, and an additional 100 mg could be given, depending on the hemodynamic response. Maintenance therapy (200 mg/day) was started on the same day, making it possible that a patient could have received as much as 400 mg of metoprolol the day of surgery. The starting dose of metoprolol used in POISE was two to eight times the commonly prescribed dose.
The initial 100-mg dose of metoprolol used in POISE has a similar beta1-receptor blockade potency compared with the 5-mg dose of bisoprolol used in DECREASE I.3 However, in DECREASE I, bisoprolol was initiated 30 days prior to surgery and was titrated, if necessary, according to heart rate. The maintenance dose of bisoprolol was half of the maintenance dose used in POISE. In the later DECREASE trials, the starting dose of bisoprolol was only 2.5 mg. Therefore, there was a huge difference in beta-blocker dosing between POISE and DECREASE.