Postoperative gastrointestinal tract dysfunction: An overview of causes and management strategies
ABSTRACT
Postoperative gastrointestinal (GI) tract dysfunction is common and has a complex, multifactorial pathogenesis. Perioperative administration of targeted amounts of fluid to optimize ventricular filling and end-organ perfusion has consistently been shown to improve mortality and other outcomes, particularly GI tract perfusion and function. The choice of fluid loading affects postoperative recovery, with colloid showing superiority over crystalloid, and lactated Ringer’s solution proving better than normal saline. Other methods of reducing postoperative GI tract dysfunction with some proven degree of success include simple, low-cost interventions such as early initiation of oral feeding, early use of laxatives, and gum chewing. There is no evidence that prophylactic nasogastric decompression accelerates return of bowel function.
KEY POINTS
- GI tract dysfunction is the most common type of postoperative morbidity and frequently delays hospital discharge.
- Low-grade hypovolemia leading to gut ischemia is a common but neglected mechanism of postoperative GI tract dysfunction.
- Administration of colloid to achieve target levels of cardiac output improves gut perfusion and lowers the incidence of GI tract dysfunction.
- Doppler-guided fluid management reduces GI morbidity and length of hospital stay in surgical patients.
Tolerance of an enteral diet is one of the fundamental components of postoperative wellness, along with the ability to mobilize freely without supplemental oxygen and a readiness to be discharged home as soon as possible. Accordingly, postoperative gastrointestinal (GI) tract dysfunction is best defined as intolerance of an enteral diet after having been tolerant of one preoperatively. I prefer the term postoperative GI tract dysfunction over postoperative ileus, as ileus is ill defined, covering a wide spectrum of clinical signs and having a range of published incidences so broad (5%–100%) that it defies useful discussion.
GI DYSFUNCTION: A COMMON POSTOPERATIVE MORBIDITY
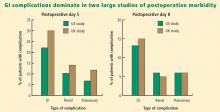
Postoperative GI tract dysfunction is common, as illustrated by a large prospective cohort study at Duke University Medical Center3 that used the Postoperative Morbidity Survey (which has since been validated4) to document complications following major noncardiac surgery (ie, anticipated duration > 2 hours and anticipated blood loss > 500 mL). Hospital discharge was delayed in 27% of the study’s 438 patients as a result of a postoperative complication, and GI dysfunction was the most common type of complication overall and on postoperative days 5, 8, and 15. Episodes of GI dysfunction ranged from intolerance of an enteral diet to ischemic gut resulting in multiple organ failure.3
A MULTIFACTORIAL PATHOGENESIS
The pathophysiology of postoperative GI tract dysfunction can be ischemic, metabolic, toxic, neurogenic, myogenic, pharmacologic, or mechanical.
It is important to recognize that in many cases no single factor explains the whole story behind postsurgical GI tract dysfunction, and none of these factors is an ipso facto cause of such dysfunction. For instance, a “mechanical” pathogenesis refers to any manipulation of the gut that causes an inflammatory response in the gut’s various layers, resulting in injury.5,6 However, GI tract dysfunction commonly occurs after operations (including laparoscopic procedures) in which the gut was not handled at all. Similarly, in terms of a pharmacologic pathophysiology, while opioids can affect GI propulsion and cause constipation,7,8 avoidance of opioid use does not ensure prevention of GI tract dysfunction. Moreover, opioid abusers do not generally exhibit intolerance of enteral nutrition.
A common mechanism that is often ignored is perioperative gut ischemia resulting in low-grade injury. Low-grade hypovolemia can cause loss of perfusion to the tip of the microvillus, triggering apoptosis and potentially necrosis, which typically requires about 3 days for recovery. An experiment among 6 healthy volunteers who underwent elective hemorrhage (25% of blood volume removed) over 1 hour demonstrated that gastric tonometry was an earlier indicator of hypovolemia than were commonly measured hemodynamic variables such as invasive blood pressure, stroke volume, heart rate, and lactate and arterial blood gas measurements.9
FLUID LOADING AIDS GI RECOVERY
A targeted increase of intravascular volume and global blood flow perioperatively has been shown repeatedly to improve surgical outcome.10–24 In clinical trials, the most common intervention to achieve the predetermined hemodynamic goal has been fluid loading. Overall, targeted increases in perioperative global blood flow have been associated with reduced mortality,25 with the presumed mechanism being maintenance of end-organ perfusion.
The role of end-organ perfusion maintenance was confirmed in a controlled study of 60 patients undergoing cardiac surgery in which perioperative fluid loading (with colloid) maintained gut perfusion as measured by gastric tonometry, whereas a control group had a reproducible reduction in gut perfusion.15 Fluid loading was associated with a significant reduction in the incidence of gut mucosal hypoperfusion—from 56% to 7%—and significant reductions in the incidence of minor and major complications, mean days in the hospital, and mean days in the intensive care unit.
Fluid type matters
The type of intraoperative fluid loading is a factor in postoperative recovery.
Colloid vs crystalloid. Moretti et al found that colloid (6% hetastarch in saline or 6% hetastarch in balanced salt) was superior to crystalloid (lactated Ringer’s solution) in preventing nausea, severe pain, vomiting, periorbital edema, and double vision postoperatively (P < .05 for all) despite comparable hemodynamic profiles.26
Ringer’s vs normal saline. Williams et al compared intravenous lactated Ringer’s solution with normal saline (0.9% sodium chloride) in a randomized study of healthy volunteers.27 The group that received normal saline demonstrated central nervous system changes and a much higher incidence of abdominal discomfort, a finding consistent with the toxic properties of chlorine to the gut.
Balanced electrolyte solutions vs saline-based fluids. Wilkes et al compared crystalloid and colloid solutions with physiologically balanced electrolyte formulations (Hextend) against saline-based fluids (Hespan) in elderly surgical patients.28 They found that balanced electrolyte solutions were superior in improving gastric mucosal perfusion and preventing hyperchloremic metabolic acidosis. As a result of a reduction in GI tract perfusion, postoperative vomiting was more frequent in the group receiving saline-based fluids.
Evidence for Doppler-guided fluid management
Use of esophageal Doppler ultrasonography to guide fluid administration intraoperatively is fairly common in the United Kingdom and is based on randomized controlled trials showing that Doppler-guided colloid administration to maximize stroke volume reduces morbidity and length of hospital stay in surgical patients. In one government-supported study of 128 colorectal resection patients, Doppler-guided small boluses of colloid increased stroke volume, cardiac output, and oxygen delivery compared with conventional (central venous pressure–based) fluid management.29 Gut function improved significantly faster with Doppler-guided fluid management as evidenced by a more rapid return of flatus, opening of bowels, and achievement of a full diet, and by faster discharge from the hospital. The incidence of GI complications was reduced from 45.3% in the conventional management group to 14.1% in the Doppler group. The relative risk of GI tract dysfunction was 5.3 times higher with conventional management.