Strategies for managing coinfection with hepatitis B virus and HIV
ABSTRACT
Hepatitis B virus (HBV) infection is more aggressive in individuals coinfected with human immunodeficiency virus (HIV): in the presence of HIV, HBV carrier rates and viremia levels are higher, episodes of activation are more frequent, cirrhosis progresses more quickly, and hepatocellular carcinoma occurs more often than with HBV infection alone. As in HBV monotherapy, the objective of treatment is suppression of viral replication. Standard or pegylated interferon may be appropriate treatment for chronic HBV infection for patients who have not yet started highly active antiretroviral therapy (HAART) for their HIV. When treatment is required for both diseases, the use of a combination of nucleoside and nucleotide analogues is prudent, with careful selection of therapy to reduce the risk of antiviral resistance—a particular concern for patients receiving antiretroviral therapy for both HIV and HBV. HBV DNA levels should be monitored every 3 months; the frequency can be extended to every 6 months once the viral load becomes stable or undetectable.
KEY POINTS
- Patients with HBV/HIV coinfection are at relatively high risk of frequent HBV activation, progression to cirrhosis, and death from liver-related causes.
- If the patient does not yet require HAART but requires treatment for HBV, this is itself an indication for HAART, since monotherapy for HBV is associated with development of resistance to HIV therapy.
- Nucleoside and nucleotide analogues should not be used as monotherapy in the HBV/HIV-coinfected patient because of the risk of inducing HIV resistance.
Worldwide, 40 million people are infected with the human immunodeficiency virus (HIV). As many as 4 million of them are coinfected with hepatitis B virus (HBV).1 In North America and Europe, the highest prevalence of HBV/HIV coinfection is in men who have sex with men. Approximately half of HIV-positive men who have sex with men have evidence of prior or active HBV infection, and 5% to 10% have chronic HBV infection. Among those who acquire HIV through injected drug use or through heterosexual transmission, the coinfection rate is much lower.2,3
Coinfection with HBV and HIV follows a different course elsewhere in the world. For example, in Africa and Asia, HBV is usually acquired first through neonatal or childhood infection, with either vertical or horizontal transmission after birth.4,5 In parts of Africa, ritual scarification is likely a major player in the adolescent transmission of HBV. (Ritual scarification is the practice of creating small incisions in the skin of adolescents and rubbing black ash in the wounds to form scars; the cutting instruments are not sterilized between rituals.)
NATURAL HISTORY
In general, HBV tends to be more aggressive in HIV-positive individuals than in monoinfected individuals,2,6 with higher HBV carrier rates, higher levels of HBV viremia, more frequent episodes of activation, and faster progression to cirrhosis.
Hepatocellular carcinoma occurs more often, its onset is earlier, and its course is more aggressive in coinfected individuals than in monoinfected individuals.7,8 Using data from a prospective cohort study, Thio et al9 found that among men coinfected with HIV and HBV, liver-related mortality was almost 19 times greater compared with men infected with HBV only and more than seven times greater compared with those infected with HIV only.
In an observational longitudinal cohort study,10 the risk of death from liver disease in HIV-positive persons was nearly three times greater among those also infected with HBV (P < .0001).
ASSESSING WHEN TO TREAT
The objectives of HBV therapy in individuals coinfected with HIV are similar to those in the population infected with HBV alone. Suppression of viral replication is the major goal. Ideally, the viral load should be reduced to an undetectable level, which will result in normalization of alanine aminotransferase (ALT) level, improved liver histology, reduced risk of progression to cirrhosis and liver failure (although supportive evidence from controlled clinical trials is lacking), and likely reduced incidence of hepatocellular carcinoma.
For those who are hepatitis B e antigen (HBeAg) positive, seroconversion may be a convenient end point for treatment, although for many patients seroconversion is not associated with remission of disease activity or viral replication.
Treatment decisions depend on whether or not the patient requires highly active antiretroviral therapy (HAART) for HIV infection. If HAART is indicated, then HIV agents that have HBV activity are incorporated into the regimen. If the patient does not yet require HAART for HIV but requires treatment for hepatitis B, this is itself an indication for HAART, since monotherapy for HBV is associated with the development of HIV resistance.
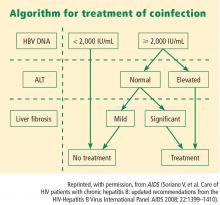
Viral load and ALT
Liver biopsy
If the ALT is normal in the presence of a high HBV DNA level, a liver biopsy is recommended, partly because the ALT level is an inadequate indicator of the severity of liver disease. If significant fibrosis is present, treatment is recommended. No treatment is required if fibrosis is mild, but liver biopsies should be repeated every 3 to 5 years in this group because a hallmark of HBV infection is its variability in time to progression. The extent of fibrosis may influence the choice of therapy.
Often in the coinfected patient, HBV-related liver injury must be distinguished from other forms of liver injury. For instance, some of the drugs used to treat HIV infection can induce nonalcoholic fatty liver disease and lipodystrophy. Because the risk of advanced fibrosis is higher in the coinfected patient than in the patient infected only with HBV, the threshold for biopsy in the coinfected patient should be lower.
At present, noninvasive tools to assess the extent of liver injury have not been validated in chronic HBV infection, unlike in hepatitis C virus infection.