The current state of antiplatelet therapy in acute coronary syndromes: The data and the real world
ABSTRACT
Managing antiplatelet therapy for patients with acute coronary syndromes (ACS) is complex, and current therapy options and approaches for these patients are suboptimal. Despite the use of available antiplatelet therapies—aspirin, clopidogrel, and the parenteral glycoprotein IIb/IIIa inhibitors—recurrence of ischemic events in patients with ACS continues to rise over time. Moreover, bleeding remains an important—and often underappreciated—risk with these therapies, and national registries demonstrate that dosing of antiplatelet therapies frequently strays from evidence-based guidelines. Recent quality-improvement initiatives developed in conjunction with national registries of patients with ACS promise to improve adherence to guidelines through hospital-specific performance reports. More evidence-based use of existing and emerging antiplatelet agents has the potential to improve both ischemic and bleeding outcomes in patients with ACS.
KEY POINTS
- Recurrent ischemic events have been observed in all antiplatelet trials to date, in spite of the addition of more potent antiplatelet regimens.
- There appears to be a gradient of benefit from dual antiplatelet therapy depending on patients’ risk of thrombotic events (the greater the risk, the greater the benefit).
- Local practice patterns in interventional therapy for ACS should be taken into consideration when applying results from clinical trials to clinical practice.
- ACS patients who stand to benefit most from antiplatelet therapies also are at the greatest risk of bleeding from those therapies.
- The importance of a tailored approach to antiplatelet therapy and dosing is becoming more widely recognized.
The final event leading to acute coronary syndromes (ACS) is spontaneous atherosclerotic plaque rupture. This event is analogous to the plaque rupture caused by percutaneous coronary intervention (PCI). Both events initiate a platelet response that starts with the adhesion of platelets to the vessel wall, followed by the activation and then aggregation of platelets.
The clinical consequences of intravascular platelet activation and aggregation are well known: death, myocardial infarction (MI), myocardial ischemia, and arrhythmias. In terms of health care burden, ACS is the primary or secondary diagnosis in 1.57 million hospitalizations annually in the United States—specifically, unstable angina or MI without ST-segment elevation in 1.24 million hospitalizations, and MI with ST-segment elevation in 330,000 hospitalizations.1
This real-world impact of ACS is tempered by the real-world use and effectiveness of our antiplatelet drug therapies, which is the focus of this article. I begin with a brief review of the evidence surrounding three major antiplatelet therapies used in ACS management—aspirin, clopidogrel, and the glycoprotein IIb/IIIa inhibitors. I then review the updated evidence-based guidelines for the use of antiplatelet therapies in ACS. I conclude with an overview of how US hospitals are actually using these therapies, with a focus on two particularly important challenges—bleeding risk and appropriate dosing—and on initiatives under way to bridge the gap between recommended antiplatelet therapy for ACS and actual clinical practice.
ANTIPLATELET THERAPY IN ACUTE CORONARY SYNDROMES
Aspirin
Although aspirin has long been the bedrock of antiplatelet therapy in patients with ACS, its effects on the heart are still being elucidated. Several placebo-controlled trials of aspirin, each with relatively few subjects, have been conducted in the setting of ACS without ST-segment elevation.2–5 Although confidence intervals were wide, these studies showed a favorable effect of aspirin relative to placebo on the risk of death and nonfatal MI.
Clopidogrel and dual antiplatelet therapy
CURE trial: prevention of recurrent events in patients with ACS. Dual antiplatelet therapy with the thienopyridine agent clopidogrel plus aspirin was investigated in patients presenting with ACS without ST-segment elevation in the landmark CURE trial (Clopidogrel in Unstable Angina to Prevent Recurrent Events).7 This study randomized 12,562 patients presenting within 24 hours of ACS symptom onset to either clopidogrel or placebo, in addition to aspirin, for 3 to 12 months. Clopidogrel was administered as a loading dose of 300 mg followed by a maintenance dosage of 75 mg/day. Randomization to clopidogrel was associated with a highly significant 20% relative reduction in the primary end point, a composite of cardiovascular death, MI, or stroke at 12 months (9.3% incidence with clopidogrel vs 11.4% with placebo; P = .00009). Despite this impressive reduction in ischemic events with clopidogrel, the cumulative event rate continued to increase over the course of the 12-month trial in both study arms. This persistent recurrence of ischemic and thrombotic events has been observed in all antiplatelet trials to date, in spite of the addition of more potent antiplatelet regimens.
Two subanalyses of the CURE results yielded further insights. One analysis examined the timing of benefit from clopidogrel, finding that benefit emerged within 24 hours of treatment and continued consistently throughout the study’s follow-up period (mean of 9 months), supporting the notion of both early and late benefit from more potent antiplatelet therapy in ACS.8 A separate subgroup analysis found that the efficacy advantage of clopidogrel plus aspirin over aspirin alone was similar regardless of whether patients were managed medically or underwent revascularization (PCI or coronary artery bypass graft surgery [CABG]).9
CHARISMA trial: prevention of events in a broad at-risk population. Several years before the CURE trial, clopidogrel was initially evaluated as monotherapy in patients with prior ischemic events in the large randomized trial known as CAPRIE (Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events), in which aspirin was the comparator.10 Rates of the primary end point—a composite of vascular death, MI, or stroke—over a mean follow-up of 1.9 years were 5.3% in patients assigned to clopidogrel versus 5.8% in those assigned to aspirin, a relative reduction of 8.7% in favor of clopidogrel (P = .043).
The CAPRIE study set the stage for CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance), which set out to determine whether dual antiplatelet therapy with clopidogrel plus aspirin conferred benefit over aspirin alone in a broad population of patients at high risk for atherothrombotic events.11 No significant additive benefit was observed with dual antiplatelet therapy in the overall CHARISMA population in terms of the composite end point of MI, stroke, or cardiovascular death over the median follow-up of 27.6 months.11
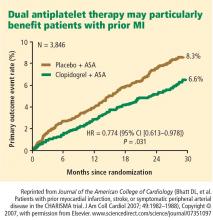
The investigators then analyzed outcomes in a large subgroup of the CHARISMA population—the 9,478 patients who had established vascular disease, ie, prior MI, stroke, or symptomatic peripheral arterial disease.12 Rates of the composite end point (MI, stroke, or cardiovascular death) in this subgroup were 7.3% with clopidogrel plus aspirin versus 8.8% with aspirin alone, representing a 1.5% absolute reduction and a 17% relative reduction with dual antiplatelet therapy (P = .01). The CHARISMA investigators concluded that there appears to be a gradient of benefit from dual antiplatelet therapy depending on the patient’s risk of thrombotic events.
Importance of longer-term therapy. Similarly, additional recent data indicate that interrupting clopidogrel therapy leads to an abrupt increase in risk among patients who experienced ACS months beforehand. Analysis of a large registry of medically treated patients and revascularized patients with ACS showed a clustering of adverse cardiovascular events in the first 90 days after clopidogrel discontinuation, an increase that was particularly pronounced in the medically treated patients.13 Like the findings from the CHARISMA subanalysis above, these data suggest that continuing clopidogrel therapy beyond 1 year may be beneficial, although the ideal duration of therapy and the patient groups most likely to benefit requires further study.