Novel antiplatelet strategies in acute coronary syndromes
ABSTRACT
Antiplatelet therapies for the treatment of acute coronary syndromes (ACS) act to interrupt various pathways of platelet activation. Clopidogrel, an established thienopyridine antiplatelet medication, inhibits adenosine diphosphate (ADP)–induced platelet aggregation to a modest degree and with wide variability in platelet response. Accumulating data suggest that a 600-mg loading dose of clopidogrel may help overcome the suboptimal response to the standard 300-mg dose seen in some patients. Prasugrel is a third-generation investigational thienopyridine that demonstrates more potent inhibition of platelet aggregation and more consistent platelet response compared with standard- and high-dose clopidogrel. A large clinical trial showed prasugrel to be superior to standard-dose clopidogrel in reducing ischemic events in patients with ACS scheduled for percutaneous coronary intervention, although prasugrel was associated with a significantly higher risk of major bleeding events. Other investigational antiplatelet agents also display more potent and consistent inhibition of platelet aggregation than is seen with clopidogrel. These include AZD6140, a reversible ADP receptor blocker; cangrelor, a rapidly acting intravenous ADP receptor blocker; and the thrombin receptor antagonist SCH 530348.
KEY POINTS
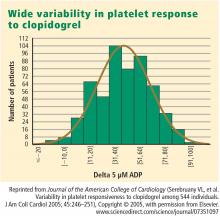
- There is substantial interpatient variability in the response to clopidogrel.
- In the large TRITON-TIMI 38 trial, the composite rate of death, myocardial infarction, or stroke was reduced by 19% and the rate of stent thrombosis was halved in patients receiving prasugrel compared with standard-dose clopidogrel.
- The risk of major bleeding with prasugrel is highest in patients aged 75 or older, those weighing less than 60 kg, and those with a history of stroke or transient ischemic attack.
- Thrombin receptor antagonists are being studied to see if their use can reduce ischemic events without increasing bleeding.
An enhanced understanding of platelet biology, as reviewed in the previous article in this supplement, has made it possible to identify a wide variety of platelet agonists. This knowledge has fostered the development of a host of pharmacologic strategies to block agonists such as cyclooxygenase, thromboxane, adenosine diphosphate (ADP), and thrombin, among others. This article will discuss the pharmacologic properties of novel antiplatelet agents, as well as alternative dosing of the established antiplatelet agent clopidogrel, and will review data from available comparative and placebo-controlled trials of these agents. The article concludes with comparative perspectives on the potential roles and relative advantages of these agents in the evolving management of patients with acute coronary syndromes (ACS).
CLOPIDOGREL AND THE CHALLENGE OF VARIABLE RESPONSE
Clopidogrel, a member of the thienopyridine class of ADP receptor inhibitors, is well established for use in patients with ACS at a loading dose of 300 mg followed by a maintenance dose of 75 mg/day. At this loading dose, inhibition of platelet aggregation to ADP is approximately 30%, and the time to peak effect is approximately 4 to 6 hours.1
As with most other drugs, the response to clopidogrel is variable. However, in contrast to the accepted measures of response to antihypertensive or lipid-lowering drugs, there are no routinely used tests for measuring response to antiplatelet therapies. As a result, a “one size fits all” strategy in the dosing of clopidogrel has prevailed.
,This variability in response is clinically relevant. In a study assessing clopidogrel responsiveness by ADP-induced platelet aggregation in 60 patients who experienced ST-segment-elevation myocardial infarction (MI), Matetzky et al found that the lowest levels of clopidogrel responsiveness were associated with a significantly elevated rate (P = .007) of recurrent cardiovascular events 6 months after the MI.3 Gurbel et al found a similar association between clopidogrel responsiveness and subacute stent thrombosis in a study of 120 patients using two different methods—light transmission aggregotomy to 5 μmol/L of ADP, and the ratio of vasodilator-stimulated phosphoprotein reactivity—to assess clopidogrel responsiveness.4
Increasing the loading dose raises response rates
One proposed method for boosting responsiveness to clopidogrel in suboptimal responders is the use of a higher dose. In a study of 190 patients undergoing coronary stenting, increasing the loading dose from 300 mg to 600 mg reduced the rate of clopidogrel resistance (defined as a < 10% absolute change in aggregation to 5 μM of ADP at 24 hours) from 28% to 8% (P < .001),5 a finding that supports the notion of enhanced response at doses up to 600 mg. Single loading doses in excess of 600 mg yield diminishing returns in terms of platelet inhibition, most likely as a result of clopidogrel pharmacokinetics.6
Compared with 300 mg of clopidogrel, the more potent platelet inhibitory effect of a 600-mg dose translated to a two-thirds reduction (P = .041) in the composite end point of death, MI, or target vessel revascularization at 30 days in a study of 255 patients with stable coronary artery disease undergoing percutaneous coronary intervention (PCI).7 The reduction in this composite end point with high-dose clopidogrel was driven by a reduction in the incidence of periprocedural MI.
In a separate study of 292 patients with non‑ST-segment-elevation ACS who were scheduled for PCI, the superior platelet response to 600 mg versus 300 mg of clopidogrel translated to a 60% reduction in adverse thrombotic events (P = .02), and this benefit extended beyond rates of periprocedural MI.8
Similar results with increased maintenance dose
Similarly, emerging data suggest that raising the maintenance dose of clopidogrel can also raise response rates. In a study of 60 patients, doubling the maintenance dose of clopidogrel after PCI from 75 mg/day to 150 mg/day resulted in improved platelet inhibition as assessed by rapid platelet function analysis.9 Likewise, a 150-mg/day maintenance dose of clopidogrel was associated with a superior antiplatelet effect compared with 75 mg/day in a study of 40 patients with type 2 diabetes.10
Large definitive trial is under way
In the wake of these smaller trials, a large randomized trial known as CURRENT is comparing a strategy of high-dose clopidogrel with standard-dose clopidogrel in patients with ACS for whom an early invasive management strategy is planned.11 The high-dose regimen involves a 600-mg loading dose followed by 150 mg/day for 1 week and then 75 mg/day for 3 weeks, whereas the standard-dose regimen involves a 300-mg loading dose followed by 75 mg/day for 4 weeks. Both groups are being further randomized to low-dose aspirin (75 to 100 mg/day) or high-dose aspirin (300 to 325 mg/day) for 30 days after PCI. With a target enrollment well beyond 10,000 patients, CURRENT should definitively clarify the relative efficacy and safety of high-dose clopidogrel in this setting.
Tailoring clopidogrel therapy
Investigators have explored tailoring the dosing of clopidogrel around the time of PCI based on the degree of platelet inhibition. In one study, administering additional loading doses of clopidogrel, up to a total of 2,400 mg, before PCI in patients with a suboptimal degree of platelet inhibition resulted in a lower rate of ischemic complications following PCI.12