Bone sarcomas: Overview of management, with a focus on surgical treatment considerations
ABSTRACT
Outcomes for patients with bone sarcomas have improved dramatically over the past 40 years, and most bone sarcomas today are treated with surgery and chemotherapy. The most common clinical findings in patients with bone sarcomas are pain and an enlarging bone mass, although pain is not generally a good indicator of malignancy. In general, any patient with a bone mass with indeterminate imaging findings should be referred to an orthopedic oncologist. Bone sarcomas are diagnosed after a biopsy, which is best performed by the surgeon who will be doing the curative resection. Postresection reconstruction of the affected limb is generally done with an allograft-prosthetic composite or a modular metallic prosthetic joint replacement device. Post-therapy follow-up at frequent and regular intervals is critical to assess for recurrence and lung metastasis.
RESECTION
For some bone sarcomas, such as osteosarcoma and Ewing sarcoma, there is a preference to treat the potential micrometastatic disease at the beginning of the course, prior to surgical treatment. This may result in reduction of the soft-tissue mass about the bone tumor and/or maturing of the mass, allowing for easier resection.
Importance of margins
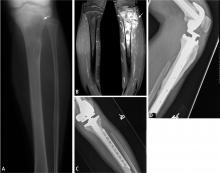
The goal of resection is to achieve a margin or normal cuff of tissue around the pseudocapsule of the tumor. In general, the larger the margin, the less the chance of recurrence.10–12 Ideally, the tumor and pseudocapsule should not be violated or exposed and a margin of at least 1 cm should be obtained. It has been postulated that margins of less than 1 cm may be associated with a very low rate of recurrence, although no well-controlled study has proven this and such a study would be difficult to perform given the rarity and heterogeneity of bone sarcomas and the variability in their assessment and surgical treatment.
Intralesional surgery is generally to be avoided
Intralesional surgery should not be performed on high-grade bone sarcomas because it will lead to a high risk of local recurrence regardless of whether the patient receives perioperative radiation therapy or chemotherapy. If intralesional surgery has been performed for a high-grade sarcoma at an outside institution, re-excision of the tumor bed is recommended, as it has reduced the rate of recurrence following intralesional surgery.13 For low-grade chondrosarcomas, intralesional curettage (ie, violating the margin of the tumor by scraping it out thoroughly) with use of an adjuvant (freezing, phenol, methylmethacrylate, or argon beam) may be adequate and has been reported to have a low rate of recurrence.14
Preoperative planning
The resection procedure involves careful preoperative planning, typically guided by an MRI reviewed by a musculoskeletal tumor radiologist. General anesthesia is usually preferred because it can be used for a lengthy procedure, ensures complete muscle relaxation over the duration of the procedure, and allows for immediate postoperative nerve assessment. If neurovascular structures are not encased (ie, not more than 50% surrounded in the case of arteries or motor nerves), these structures are spared. If arteries are encased, arterial resection with reverse interpositional vein graft, synthetic graft, or vein allograft allows for bypass of the vessel and leaves the encased structure with the resection specimen for en bloc resection. In Ewing sarcoma, if the tumor is adjacent to but not encasing the neurovascular structures, the radiation oncologist is consulted about whether there is a preference for pre- or postoperative radiation therapy.
Limb salvage for Ewing sarcoma was originally with radiation only, but subsequently limb-salvaging surgery has been shown in several studies to have lower rates of local failure.6,15–18 Whether primary radiation or surgery is performed after the initiation of chemotherapy is generally determined by a discussion between the surgeon and radiation oncologist about the feasibility of a negative margin with surgery and the inherent functional loss with resection. There are particular concerns about radiation in younger patients, who have a relatively high rate of postradiation sarcoma.18
In osteosarcoma and chondrosarcoma, radiation has been found not to be effective, so resection with a negative margin is especially important for preventing local recurrence.
RECONSTRUCTION
Allograft or metallic prosthesis?
In the proximal and distal femur, modular metallic replacement prosthetic joint devices are used. Often a wafer of greater trochanter bone (if uninvolved in the tumor process) can be preserved and a “cable-claw” attachment to the metal component can be accomplished instead of using an allograft.
Since the proximal humerus is not weight-bearing and because of the importance of the rotator cuff, use of an APC in the proximal humerus can be most helpful. Function is not good with a metallic proximal humerus implant alone, and the dislocation rate is high over long-term follow-up, owing to lack of healing of the rotator cuff remnant to the metal prosthesis.
In patients with scapular sarcomas, allograft or prosthetic reconstruction has not been consistently better than simply repairing the remaining muscles to each other, so we generally do not use allografts or prostheses after sarcoma resection in these patients.
Growing bones of youth pose special challenges
In growing children, who represent a large share of bone sarcoma patients, reconstruction after resection in the lower extremity is challenging, particularly in terms of addressing leg length inequality. In general, a prosthesis is used and if the end growth discrepancy will be greater than 3 cm, use of an expandable prosthesis is considered. Use of these expandable prostheses has been fraught with complications, however, and by their nature they require revision because of breakage. An alternative is reoperation to disconnect the modular prosthesis and insert an additional 1- to 2-cm segment to increase length when necessary. Allograft bones are a common method of reconstruction when the resection does not involve the joint.
Rotationplasty
Rotationplasty—which involves saving the portion of the extremity distal to the resection site and reattaching it after being rotated 180 degrees—is rarely performed for leg reconstruction, in light of the disfiguring nature of the surgery as a result of the 180-degree rotation.
When rotationplasty is performed, the lower tibia and foot generally are brought up to the middle or proximal femoral area and attached to the short proximal femur. Rather than a short above-knee amputation, the reversed foot functions as a knee, allowing for better prosthetic function (ideally similar to a short below-knee prosthesis), and adds length to a short above-knee amputation.
Another alternative is a tibial turn-up to add length to a very short above-knee amputation if the vessels are not involved with the tumor and limb salvage is otherwise not practical. In this procedure the ankle can be turned up to the hip and the proximal tibia ends up distal to the ankle.