Cardiac amyloidosis: An update on diagnosis and treatment
ABSTRACT
Cardiac amyloidosis (CA), once thought to be a rare disease, is increasingly recognized due to enhanced clinical awareness and better diagnostic imaging. CA is becoming of heightened interest to the cardiology community given more effective treatment strategies for light chain amyloidosis (AL), as well as emerging therapies for transthyretin amyloidosis (ATTR). Furthermore, reversing amyloid deposition in affected organs using monoclonal antibodies is actively being tested in clinical trials. A high index of suspicion and a systematic approach to the diagnosis of CA can lead to referral to a center of expertise for timely treatment.
KEY POINTS
- AL and ATTR are the 2 main types of amyloidosis that affect the heart.
- Serum and urine protein electrophoresis are inadequate laboratory tests to screen for AL given low sensitivity, and should be replaced by the serum free light chain assay as well as immunofixation of the serum and urine.
- AL cardiac amyloidosis (AL-CA) requires timely diagnosis and referral to hematology due to high mortality without prompt treatment.
- 99mTechnetium pyrophosphate bone scintigraphy is an affordable, noninvasive tool that has revolutionized the diagnosis of ATTR cardiac amyloidosis (ATTR-CA).
- The US Food and Drug Administration will likely approve new therapies for ATTR in late 2018.
Advanced noninvasive diagnostic tools for CA
Over the past decade, the ability to diagnose CA noninvasively has dramatically improved with strain imaging using 2D speckle tracking echocardiography, cardiac magnetic resonance imaging (MRI), and nuclear bone scintigraphy. These diagnostic tools have given clinicians options to pursue the diagnosis of CA without directly proceeding to endomyocardial biopsy.
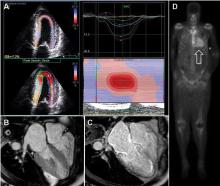
Cardiac MRI. Cardiac MRI is useful for the diagnosis of CA (Figure 4B, 4C). Imaging after administration of gadolinium contrast shows a characteristic late gadolinium enhancement (LGE) pattern that is diffuse and subendocardial, and does not follow any particular coronary distribution.49 LGE can also be seen in the right ventricle and the atrial walls, and can be transmural and patchy in ATTRwt-CA. This pattern is highly sensitive (93%) and specific (70%) for CA with an overall negative predictive accuracy of 84%.50
One of the main limitations of cardiac MRI for the diagnosis of CA is the inability to give contrast in patients with reduced glomerular filtration rate. However, native T1-myocardial mapping techniques that do not require contrast show significantly increased native T1 times in CA and offer a promising alternative. Cardiac MRI parameters such as LGE, the difference in inversion time between the LV cavity and myocardium, native T1 mapping, and extracellular volume offer prognostic information. A greater than fivefold mortality increase is seen in CA patients with transmural LGE compared with those without LGE.49,50
99mTcPYP scintigraphy. 99mTcPYP, a radiotracer used in bone scans, was initially used in cardiology to quantify myocardial infarction due to its ability to localize calcium.51 Its potential utility in CA came in 1982 when diffuse myocardial 99mTcPYP uptake on cardiac radionucleotide imaging was noted in 10 patients with tissue-proven amyloidosis.52 Several subsequent studies reproduced and expanded upon this observation and revealed its diagnostic value, specifically showing that there is significant uptake in ATTR-CA and no to mild uptake in AL-CA. This offers a significant advantage over other noninvasive modalities in that it not only confirms the diagnosis of CA but differentiates ATTR-CA.16
99mTcPYP myocardial radiotracer uptake is graded by the semiquantitative visual score of cardiac retention, where grade 0 = no cardiac uptake, grade 1 = mild uptake less than bone, grade 2 = moderate uptake equal to bone, and grade 3 = high uptake greater than bone (Figure 4D).42 Additionally, quantitative analysis of heart retention can be calculated drawing circular regions of interest over the heart and mirrored on the contralateral chest wall. A heart-to-contralateral ratio greater than 1.5 is consistent with the diagnosis of ATTR-CA.53,54 In 2016, a multicenter study showed that grade 2 or 3 myocardial radiotracer uptake on bone scintigraphy in the absence of evidence of a monoclonal gammopathy was diagnostic for ATTR-CA, providing a cost-effective and non-invasive technique with a specificity and positive predictive value of 100% (confidence interval, 99.0–100%).42
Endomyocardial biopsy, right heart catheterization, and fat biopsy
Endomyocardial biopsy is essentially 100% sensitive for the diagnosis of CA.25 The main risk of pursuing endomyocardial biopsy is about a 1% risk of right ventricular perforation leading to cardiac tamponade.55 The other limitation to this approach is that not all centers are equipped to perform this procedure. Birefringence under polarized light microscopy is histopathologically diagnostic of CA; however, further subtyping by the pathologist to determine if it is AL or ATTR is absolutely crucial. Subtyping can be performed by immunohistochemistry with caution taken for misinterpretation. If there is any question of accuracy, the specimen should be sent for laser microdissection and mass spectroscopy for accurate identification of the precursor protein type (some centers routinely perform mass spectroscopy on all myocardial specimens).16,31
Right heart catheterization is nonspecific and shows restrictive hemodynamics. The right atrial waveform shows rapid x and y descents and the right ventriclular tracing may show a dip-and-plateau pattern typical of restrictive cardiomyopathy. Cardiac output can be preserved but more commonly is low.16,31
Fat pad biopsy is 60% to 80% sensitive in AL, 65% to 85% sensitive in ATTRm, and only 14% sensitive in ATTRwt, with the accuracy dependent on the operator, pathologist, and how much tissue is removed (fat pad aspirate vs biopsy specimen).56–58 Fat pad biopsy has diagnostic limitations, and a negative fat pad biopsy does not rule out amyloidosis.
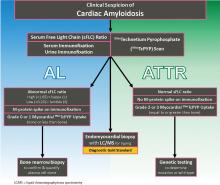
DIAGNOSTIC ALGORITHM FOR CARDIAC AMYLOIDOSIS
In conjunction with laboratory tests, 99mTcPYP scan of the heart can be ordered to investigate the possibility of ATTR-CA. Grade 2 to 3 myocardial uptake in the absence of a monoclonal plasma cell process is consistent with the diagnosis of ATTR-CA. Grade 0 or 1 myocardial uptake on 99mTcPYP scan with an abnormal sFLC ratio or positive M protein on immunofixation suggests AL-CA and a bone marrow biopsy should be performed. If the patient has an abnormal sFLC ratio and grade 2 to 3 uptake on 99mTcPYP scan, the diagnosis of ATTR-CA with unrelated monoclonal gammopathy of undetermined significance should be considered. However, this would need to be reconciled by pursuing endomyocardial biopsy and accurate tissue typing. If the 99mTcPYP scan is negative, and the sFLC ratio is normal, and immunofixation is negative, a diagnosis of CA is very unlikely.16,31,40,42–47
If the diagnosis of ATTR-CA is made, genetic testing can determine the presence or absence of a mutation to differentiate ATTRm or ATTRwt, respectively. If the diagnosis of AL-CA is suggested, a bone marrow biopsy is necessary to identify and quantify the plasma cell clone.16,31,40,42–47
TREATMENT
Management of heart failure in cardiac amyloidosis
The main treatment of heart failure revolves around sodium restriction and diuretics to relieve congestion. This can prove challenging in many patients due to the narrow window between too high or too low filling pressures. A combination of loop diuretics and an aldosterone antagonist is most effective.31,34,44,45 Torsemide is preferred over furosemide due to its superior bioavailability and longer duration of action, particularly since these patients have issues with gut edema and GI absorption. Due to dependence of the cardiac output on heart rate and the tendency for orthostatic hypotension, traditional neurohormonal antagonists including beta blockers and angiotensin-converting enzyme inhibitors are neither effective nor well tolerated.60 However, in patients with atrial fibrillation, beta blockers may need to be used for rate control. Nondihydropyridine calcium channel blockers bind avidly to amyloid fibrils and are contraindicated due to risk of profound hypotension and syncope.30,31,34,44,45 Digoxin is usually avoided in CA due to concerns of increased risk of toxicity; however, it may be used with caution for rate control in atrial fibrillation given its lack of negative inotropy.61 Maintenance of normal sinus rhythm is preferable due to the importance of atrial contribution to cardiac output.
Anticoagulation in patients with atrial fibrillation and even in patients with normal sinus rhythm and poor atrial function is important due to the high risk of thromboembolic complications.62 Pacemakers are indicated for heart block or symptomatic bradycardia.63 The role of intracardiac defibrillators is controversial, but may be warranted in selected patients with AL-CA.64,65