Cardiac amyloidosis: An update on diagnosis and treatment
ABSTRACT
Cardiac amyloidosis (CA), once thought to be a rare disease, is increasingly recognized due to enhanced clinical awareness and better diagnostic imaging. CA is becoming of heightened interest to the cardiology community given more effective treatment strategies for light chain amyloidosis (AL), as well as emerging therapies for transthyretin amyloidosis (ATTR). Furthermore, reversing amyloid deposition in affected organs using monoclonal antibodies is actively being tested in clinical trials. A high index of suspicion and a systematic approach to the diagnosis of CA can lead to referral to a center of expertise for timely treatment.
KEY POINTS
- AL and ATTR are the 2 main types of amyloidosis that affect the heart.
- Serum and urine protein electrophoresis are inadequate laboratory tests to screen for AL given low sensitivity, and should be replaced by the serum free light chain assay as well as immunofixation of the serum and urine.
- AL cardiac amyloidosis (AL-CA) requires timely diagnosis and referral to hematology due to high mortality without prompt treatment.
- 99mTechnetium pyrophosphate bone scintigraphy is an affordable, noninvasive tool that has revolutionized the diagnosis of ATTR cardiac amyloidosis (ATTR-CA).
- The US Food and Drug Administration will likely approve new therapies for ATTR in late 2018.
CLINICAL PRESENTATION
Patients with CA typically exhibit heart failure with preserved ejection fraction (otherwise known as diastolic heart failure). Dyspnea on exertion is common; however, some patients can present with more right-sided heart failure symptoms such as lower-extremity edema and ascites. Fatigue and weakness are related to low cardiac output and often attributed to nonspecific symptoms of aging. Because of the thickened ventricles, patients can often be misdiagnosed as having HCM with or without obstruction.7,34 The first manifestation of CA may be atrial fibrillation, most commonly in ATTRwt-CA, or cardioembolic stroke. Atrial fibrillation can be present for years before CA is considered. Bundle branch block and complete heart block (more common in ATTR-CA than AL-CA) may lead to pacemaker implantation.30 Angina with normal coronaries can occur, and a rare presentation may be cardiogenic shock due to diffuse ischemia.31–33 Elderly patients with CA can present with low-flow, low-gradient aortic stenosis.37
DIAGNOSIS
ECG
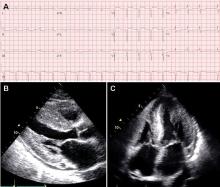
As opposed to that seen in true left ventricular hypertrophy (LVH), which leads to increased voltage on ECG, amyloid infiltration of the myocardium leads to lower voltage. Thus, what is indicative of LVH on echocardiogram combined with low voltage on the ECG is a classic finding for CA. However, only about 50% of patients with AL-CA and about 30% of patients with ATTR-CA meet true low-voltage criteria (QRS amplitude less than 5 mm in limb leads or less than 10 mm in precordial leads).30,38 Hence, the absence of low-voltage criteria does not exclude the diagnosis of CA. Approximately 10% of patients with CA confirmed by biopsy met ECG criteria for LVH.38 The key point is to consider the overall degree of voltage on the ECG relative to the degree of LV thickening on the echocardiogram, recognizing that lower voltage than what would be expected may indicate possible infiltrative disease such as CA. The other main finding on the ECG in patients with CA is a pseudoinfarct pattern with Q waves in the early precordial leads mimicking a prior anteroseptal myocardial infarction.38,39 This finding is seen in about 50% of patients (Figure 3).39 Wide QRS complexes are more frequent in ATTR-CA and lower limb voltages are more frequent in AL-CA.30
Echocardiogram
The echocardiographic finding of LVH in patients with CA is misleading in that the LV thickening is due to infiltrating amyloid fibrils and not to myocyte hypertrophy. That said, the terms LVH and LV thickening are used interchangeably when describing the echocardiographic phenotype. LV wall thickness greater than 12 mm (6 mm to 10 mm is normal) in the absence of hypertension should prompt suspicion for CA.34 LV thickening most often appears symmetric; however, occasionally it may exhibit asymmetric septal hypertrophy, particularly in ATTRwt-CA. In some cases, there may be a smaller subset that can actually have dynamic LV outflow obstruction similar to that seen in hypertrophic obstructive cardiomyopathy.22–24 An important echocardiographic clue that can differentiate CA from other diseases is thickening of both the LV and right ventricle (Figure 3). Septal wall thickness and LV mass index are greater in ATTR-CA compared with AL-CA.30 On average, the LV septum is around 15 mm in AL-CA and around 18 mm in ATTRwt-CA.40 Historically, the characteristic myocardial “granular sparking” or “speckling” pattern has low sensitivity and specificity.16 The left ventricle is not dilated; rather, the ventricular dimensions are usually smaller than normal. Although ejection fraction is usually preserved, cardiac output is low due to decreased ventricular volume.37 Systolic dysfunction occurs late in the disease.16 Diastolic dysfunction is universal, with a mitral inflow pattern that can range from stage I (abnormal relaxation) in early disease to stage III (restrictive filling pattern) in more advanced disease. Septal and lateral tissue Doppler velocities are very low in amyloid heart disease.41 Another echocardiographic clue to diagnosis is thickening of the heart valves, which is not seen in hypertensive heart disease or hypertrophic cardiomyopathy. Biatrial dilation is common, and there can be thickening of the interatrial septum.
Laboratory testing
N-terminal pro-b-type natriuretic peptide (NT-proBNP) is universally elevated in CA and is typically higher in AL-CA than in ATTR-CA. Troponin T or troponin I or both may be chronically elevated in CA and likely signify small-vessel ischemia. In the appropriate clinical context of a thickened ventricle and heart failure, an elevated troponin value (outside of an acute coronary syndrome) should trigger suspicion for CA. Workup for a monoclonal protein process should always be done when considering CA to rule out AL. Serum and urine protein electrophoresis are insensitive tests to detect AL and should not be relied upon as a screening test. The serum free light chain (sFLC) assay, which measures free kappa and lambda light chain levels and reports the ratio, is a sensitive test that should be measured routinely along with immunofixation of the serum and urine. In AL, sFLC will reveal an abnormal kappa-lambda ratio. An abnormally low ratio (less than 0.26) suggests a monoclonal lambda light chain process, while an abnormally high ratio (greater than 1.65) suggests a monoclonal kappa light chain process. Immunofixation will reveal an M-protein. Because light chains are excreted by the kidney, the serum levels of both kappa and lambda will be elevated in renal dysfunction, but the ratio should remain normal.16,31,34,40,42–47