Reproductive planning for women after solid-organ transplant
ABSTRACT
Women who receive transplants require contraception counseling because of the teratogenicity of immunosuppressant medications and the risks posed by pregnancy after transplant. Fortunately, pregnancy can succeed with careful planning and monitoring.
KEY POINTS
- The number of solid-organ transplants in US women of childbearing age has increased over the past 20 years.
- Women should wait at least 1 year after receiving a solid-organ transplant before attempting to become pregnant, and then should do so only when cleared by the transplant team and obstetrician, with close monitoring.
- The various types of contraception can be grouped by their effectiveness and by the medical eligibility criteria set by the US Centers for Disease Control and Prevention.
- Transplant recipients of childbearing age should use 2 contraceptive methods concurrently, one of which should be condoms.
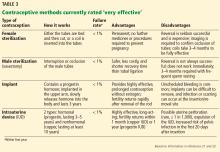
VERY EFFECTIVE CONTRACEPTIVES (UNINTENDED PREGNANCY RATE 0%–0.9%)
This tier of contraception is the most effective regardless of the patient’s adherence; it includes long-acting, reversible contraceptives and permanent sterilization (both male and female) (Table 3).21–23
Long-acting reversible contraceptives include intrauterine devices (IUDs) and the subdermal etonogestrel implant. Given their efficacy and favorable safety profile, long-acting reversible contraceptives are being promoted for use in women who have chronic medical conditions, such as transplants.24
Intrauterine devices
IUDs are long-acting and reversible. They can be used by women who are nulliparous and those of all ages, including adolescents.22
Two types of IUDs are available in the United States: nonhormonal (copper) and hormonal (levonorgestrel). The copper IUD is effective for at least 10 years, whereas the levonorgestrel IUDs last for 3 to 5 years.22
Four levonorgestrel IUDs are currently available in the United States. Their sizes and doses vary: Mirena (52 µg), Skyla (13.5 µg), Liletta (52 µg), and Kyleena (19.5 µg).
Fewer than 1% of women become pregnant in the first year of IUD use.22,23 IUDs are an ideal option for women with solid-organ transplants because they are so effective and because the patient does not have to do anything once the IUD is in.22–24 The levonorgestrel IUD Mirena has the additional advantage of reducing heavy menstrual bleeding and is currently the only hormonal IUD with FDA approval for the management of menorrhagia.
About 12% of women in the general population use IUDs as their contraceptive method of choice,25 whereas after solid-organ transplantation about 15% to 20% of women do.26
Two historic concerns regarding IUDs may explain their low rate of use in transplant recipients.
First, IUDs were believed to be less effective in women on immunosuppressive drugs because IUDs act by inducing a local inflammatory reaction. However, IUDs involve macrophage activation, which is independent of the immune processes modified by immunosuppressants (primarily T-cell function).27 A recent pilot study showed a strong inflammatory reaction in the endometrium of transplant recipients after levonorgestrel IUD insertion.28
Second, there was concern about the increased risk of pelvic inflammatory disease with IUDs, but studies have shown levonorgestrel IUDs to be safe in transplant patients.29,30
The CDC21 lists copper and levonorgestrel IUDs in MEC category 3 (the risks generally outweigh the advantages) for initiation in patients with complicated transplants and in category 2 (advantages generally outweigh the risks) in patients with uncomplicated organ transplants. The devices are in category 2 for both complicated and uncomplicated cases if the IUD is already in place.
Subdermal implant
A subdermal implant consisting of a single rod containing 68 mg of etonogestrel is commercially available in the United States. It is one of the most effective contraceptive methods, with the lowest rates of pregnancy—less than 1% per year, with protection lasting at least 3 years.22,23 This low risk makes the subdermal implant a suitable method of contraception after transplant. Daily compliance is not required, and there are no hepatic first-pass effects, which results in higher bioavailability and less chance of drug interactions.
The main disadvantage of the subdermal implant and IUDs is unscheduled bleeding. An important benefit is prolonged amenorrhea, not only for patient convenience, but for reduction of endometrial cancer risk. Insertion and removal of the implant are considered minor office procedures. The implants are classified as US MEC category 2 in uncomplicated cases; initiation in complicated cases is considered category 3 but continuation is considered category 2.21
Permanent sterilization
Permanent sterilization is another option for women and men. In women, the fallopian tubes can be occluded with a coil system implanted vaginally through a hysteroscope, or they can be severed, tied, or clamped in a laparoscopic procedure or during cesarean delivery. Pregnancy rates after tubal ligation are less than 1%,23,31 although concern exists for high failure rates with the hysteroscopic method.
Because younger patients are more likely than older patients to subsequently regret having the procedure done, all available contraceptive options should be discussed with them.31
For men, permanent sterilization is done by vasectomy, which has less associated risk and cost compared with sterilization for women.
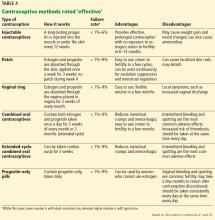
EFFECTIVE CONTRACEPTIVE METHODS (UNINTENDED PREGNANCY RATE 1%–9%)
Effective contraceptive methods, the next tier down from very effective methods, include injectable contraceptives, combined hormonal contraceptives, and progestin-only contraceptives (Table 4).
Injectable contraceptives
Depot medroxyprogesterone acetate is an injectable progestin-only contraceptive that carries a pregnancy risk of 6% with typical use and less than 1% with correct use.23 Thus, some failures are due to patients not returning for follow-up, but in some patients this method is not effective. Injections are given intramuscularly once every 3 months, avoiding the need for daily use.
A valid concern for transplant patients is that medroxyprogesterone acetate reduces bone mineral density. Although the bone effects are reversible in healthy adult women, caution is needed when prescribing this option to transplant patients who are already at increased risk of bone disease attributable to renal osteodystrophy and chronic corticosteroid use. 32,33
Recently, a subcutaneous formulation of depot medroxyprogesterone acetate (104 mg)was added to the WHO MEC for contraceptive use.34,35 The recommendations for the subcutaneous form are similar to those for the intramuscular form. In healthy women, the subcutaneous formulation is as safe and effective as the intramuscular form,36 but its efficacy after solid-organ transplant has not been determined. Both forms of depot medroxyprogesterone acetate are category 2 in the US MEC for both complicated and uncomplicated transplant cases.21