Combined hormonal contraceptives and migraine: An update on the evidence
ABSTRACT
Combined hormonal contraceptives are contraindicated in women who have migraine with aura, in whom these drugs can increase the risk of ischemic stroke. However, this contraindication is based on data from the 1960s and 1970s, when oral contraceptives contained much higher doses of estrogen. Stroke risk is not significantly increased with today’s preparations, many of which contain less than 30 μg of ethinyl estradiol. Further, in continuous regimens, ultra-low-dose formulations—those that contain less than 20 µg of ethinyl estradiol—may help prevent menstrual migraine and reduce the frequency of aura.
KEY POINTS
- There is no restriction on the use of combined hormonal contraceptives by women with migraine without aura, and the risk vs benefit for women with aura is debatable.
- Migraine with aura—but not migraine without aura—is associated with a twofold increased risk of ischemic stroke, although the absolute risk is small in healthy women who do not smoke.
- Combined hormonal contraceptives are associated with ischemic stroke, but the risk is dose-dependent. Ultra-low-dose formulations (containing ≤ 20 μg of ethinyl estradiol) do not pose an increased risk of stroke in healthy nonsmokers.
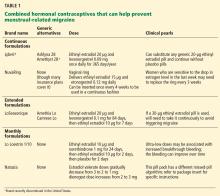
Continuous regimens: Who needs a menstrual cycle anyway?
Of note: ultra-low-estrogen combined hormonal contraceptives that have placebo intervals may not inhibit ovulation consistently in all women.32 Contraceptive efficacy is still maintained, as contraception does not require inhibition of ovulation. Other mechanisms such as thickening of cervical mucus help with pregnancy prevention.
However, if ovulation is not inhibited, the consequent postovulatory decline in estrogen will continue to contribute to estrogen-withdrawal migraine.33,34 Reducing the number of placebo days may help inhibit ovulation. Adding back adequate estrogen during the placebo break (eg, either 0.9 mg conjugated equine estrogen with a 20-µg ethinyl estradiol combined oral contraceptive, or 0.075 mg transdermal 17B estradiol with a 15-µg combined hormonal contraceptive) can prevent these migraines.33,34
Some extended-cycle regimens, which give 4 withdrawal bleeds per year, will likewise prevent estrogen-withdrawal migraine if the decline in estrogen is limited to 10 µg (Table 1). Unfortunately, most extended regimens (Seasonale, Seasonique, and their generics) entail a 20- or 30-µg drop.
Continuous or extended-cycle regimens can be prescribed using any generic 20-µg combined hormonal contraceptive that the patient tolerates, along with specific instructions on the prescription to take the pills in a continuous fashion, eg, “Do not take the placebo pills; start the next pill pack immediately after 21 days.”
Postmenopausal hormone therapy
Neither smoking nor migraine is a contraindication to the use of postmenopausal hormone therapy, which is substantially lower in dosage than combined hormonal contraceptives.
ADVISING PATIENTS ON RISKS VS BENEFITS
It is important to remember that the risks of unintended pregnancy are always greater than the risks of any contraceptive, especially in women with chronic medical conditions, including those who have migraine with aura. Other benefits include the following:
Lower mortality risk. A 2010 analysis demonstrated that in nearly 46,000 women followed since 1968, those taking combined oral contraceptives had statistically significantly lower death rates from any cause and a lower risk of death from cancer and cardiovascular diseases than women who had never taken combined oral contraceptives.36
Stroke. Though the absolute risk of stroke to an individual woman taking a low-dose or ultra-low-dose combined hormonal contraceptive has been shown to be similar to that in women who are not taking combined hormonal contraception, its impact on an otherwise healthy woman could be devastating. Clinicians must remember that current guidelines still caution against prescribing combined hormonal contraceptives in women with migraine with aura and thus should counsel their patients accordingly and document the discussion in the medical record.
Noncontraceptive benefits. Women may be prescribed a combined hormonal contraceptive for benefits beyond contraception. The obvious reasons include beneficial effects on endometriosis, anemia, acne, hirsutism, dysmenorrhea, and prevention of ovarian cysts. But other important major benefits2 include substantial reductions in the risk of ovarian cancer (> 50% decrease after 10 years)37 and endometrial cancer (additional 24% reduction for each 5 years of use),38 and a modest decrease in the risk of colon cancer (37% less risk in ever-users).39 Further, combined oral contraceptive use has been associated with a decrease in mortality rates,40,41 with no increased risk of nonreproductive cancers.41
Ultra-low-dose, continuous formulations may benefit women by decreasing the frequency of migraine with aura and menstrual-related migraine. There is no evidence that reducing aura frequency also reduces stroke risk, but this represents an important area for future research.
WHAT WOULD WE DO?
For a patient who has a history of migraine with aura, if the goal is only to prevent pregnancy, we would recommend another contraceptive option that does not involve estrogen. However, we would consider prescribing a combined hormonal contraceptive in a low-dose regimen if the patient prefers this regimen for other health benefits (eg, acne control), if she has no other risk factors for stroke, and if she gives her informed consent after a discussion of the risks and benefits. Women who have menstrual-related migraine refractory to or who cannot tolerate other migraine therapies are often willing to try a low-dose estrogen-containing contraceptive for control of their migraine, especially if they have tried it in the past and believe that it helped prevent migraine. Patients should have follow-up within 3 months to discuss whether they have benefited from the regimen in terms of headache frequency or severity.