Autosomal dominant polycystic kidney disease and the heart and brain
ABSTRACT
Autosomal dominant polycystic kidney disease (ADPKD) has numerous systemic manifestations and complications. This article gives an overview of hypertension, cardiac complications, and intracranial aneurysms in ADPKD, their pathophysiology, and recent developments in their management.
KEY POINTS
- Hypertension and left ventricular hypertrophy are common complications of ADPKD.
- Cardiovascular disease is a major cause of morbidity and death in ADPKD.
- Early diagnosis and aggressive management of high blood pressure, specifically with agents that block the renin-angiotensin-aldosterone system, are necessary to prevent left ventricular hypertrophy and progression of renal failure in ADPKD.
- Timely screening and intervention for intracranial aneurysm would lessen the rates of morbidity and death from intracranial hemorrhage.
MANAGING LEFT VENTRICULAR HYPERTROPHY IN ADPKD
Preventing and halting progression of left ventricular hypertrophy primarily involves effective blood pressure control, especially in the early stages of ADPKD (Figure 2).
A 7-year prospective randomized trial in ADPKD patients with established hypertension and left ventricular hypertrophy proved that aggressive (< 120/80 mm Hg) compared with standard blood pressure control (135–140/85–90 mm Hg) significantly reduces left ventricular mass index. ACE inhibitors were preferred over calcium channel blockers.58
HALT-PKD Study A showed that a significant decrease in left ventricular mass index can be achieved by aggressive blood pressure control (95–110/60–75 mm Hg) with an ACE inhibitor alone or in combination with an ARB in the early stages of ADPKD with preserved renal function.43
A 5-year randomized clinical trial in children with borderline hypertension treated with an ACE inhibitor for effective control of blood pressure showed no change in left ventricular mass index or renal function.36,59
These results support starting ACE inhibitor therapy early in the disease process when blood pressure is still normal or borderline to prevent the progression of left ventricular hypertrophy or worsening kidney function.
Since FGF23 is directly involved in the causation of left ventricular hypertrophy, FGF receptors may be potential therapeutic targets to prevent left ventricular hypertrophy in ADPKD. An FGF receptor blocker was shown to decrease left ventricular hypertrophy in rats with chronic kidney disease without affecting blood pressure.55
INTRACRANIAL ANEURYSM IN ADPKD
Intracranial aneurysm is the most dangerous complication of ADPKD. When an aneurysm ruptures, the mortality rate is 4% to 7%, and 50% of survivors are left with residual neurologic deficits.5,60,61
In various studies, the prevalence of intracranial aneurysm in ADPKD ranged from 4% to 41.2%, compared with 1% in the general population.5,62,63 On follow-up ranging from 18 months to about 10 years, the incidence of new intracranial aneurysm was 2.6% to 13.3% in patients with previously normal findings on magnetic resonance angiography and 25% in patients with a history of intracranial aneurysm.62,64,65
The most common sites are the middle cerebral artery (45%), internal carotid artery (40.5%), and anterior communicating artery (35.1%).66 (The numbers add up to more than 100% because some patients have aneurysms in more than 1 site.) The mean size of a ruptured aneurysm was 6 mm per a recent systematic review.66 Intracranial aneurysms 6 mm or larger are at highest risk of rupture.66
SCREENING FOR INTRACRANIAL ANEURYSM
Timely screening and intervention for intracranial aneurysm is crucial to prevent death from intracranial hemorrhage.
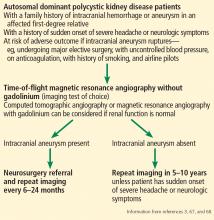
Currently, there are no standard guidelines for screening and follow-up of intracranial aneurysm in ADPKD patients. However, some recommendations are available from the ADPKD Kidney Disease Improving Global Outcomes Controversies Conference3 and Kidney Health Australia—Caring for Australasians With Renal Impairment ADPKD guidelines67 (Figure 3).
Imaging tests
Magnetic resonance angiography (MRA) with gadolinium enhancement and computed tomographic angiography (CTA) are recommended for screening in ADPKD patients with normal renal function,67 but time-of-flight MRA without gadolinium is the imaging test of choice because it is noninvasive and poses no risk of nephrotoxicity or contrast allergy.3,68 Further, gadolinium should be avoided in patients whose eGFR is 30 mL/min/1.73 m2 or less because of risk of nephrogenic systemic sclerosis and fibrosis.67,68
The sensitivity of time-of-flight MRA screening for intracranial aneurysm varies depending on the size of aneurysm; 67% for those less than 3 mm, 79% for those 3 to 5 mm, and 95% for those larger than 5 mm.69 The sensitivity of CTA screening is 95% for aneurysms larger than 7 mm and 53% for those measuring 2 mm.70,71 The specificity of CTA screening was reported to be 98.9% overall.71
When to screen
Screening for intracranial aneurysm is recommended at the time of ADPKD diagnosis for all high-risk patients, ie, those who have a family history of intracranial hemorrhage or aneurysm in an affected first-degree relative.67 It is also recommended for ADPKD patients with a history of sudden-onset severe headache or neurologic symptoms.67 A third group for whom screening is recommended is ADPKD patients who have no family history of intracranial aneurysm or hemorrhage but who are at risk of poor outcome if an intracranial aneurysm ruptures (eg, those undergoing major elective surgery, with uncontrolled blood pressure, on anticoagulation, with a history of or current smoking, and airline pilots).67
Patients found to have an intracranial aneurysm on screening should be referred to a neurosurgeon and should undergo repeat MRA or CTA imaging every 6 to 24 months.3 High-risk ADPKD patients with normal findings on initial screening should have repeat MRA or CTA screening in 5 to 10 years unless they suffer from sudden-onset severe headache or neurologic symptoms.65,67
Both smoking and high blood pressure increase the risk of formation and growth of intracranial aneurysm. Hence, meticulous control of blood pressure and smoking cessation are recommended in ADPKD patients.3,67
CARDIAC VALVULAR ABNORMALITIES IN ADPKD
Of the valvular abnormalities that complicate ADPKD, the more common ones are mitral valve prolapse and mitral and aortic regurgitation. The less common ones are tricuspid valve prolapse and tricuspid regurgitation.72–74
The pathophysiology underlying these valvular abnormalities is unclear. However, defective collagen synthesis and myxomatous degeneration have been demonstrated in histopathologic examination of affected valvular tissue.75 Also, ACE gene polymorphism, especially the DD genotype, has been shown to be associated with cardiac valvular calcifications and valvular insufficiency.57
Lumiaho et al72 found a higher prevalence of mitral valve prolapse, mitral regurgitation, and left ventricular hypertrophy in patients with ADPKD type 1 (due to abnormalities in PDK1) than in unaffected family members and healthy controls. The investigators speculated that mitral regurgitation is caused by the high blood pressure observed in ADPKD type 1 patients, since hypertension causes left ventricular hypertrophy and left ventricular dilatation. The severity of renal failure was related to mitral regurgitation but not mitral valve prolapse.
Similarly, Gabow et al24 showed that there is no significant relationship between mitral valve prolapse and progression of renal disease in ADPKD.
Interestingly, Fick et al20 found that mitral valve prolapse has no significant effect on cardiovascular mortality.