The ABCs of managing systolic heart failure: Past, present, and future
ABSTRACTHeart failure management is complex and constantly evolving. The American College of Cardiology and the American Heart Association (ACC/AHA) last issued evidence-based guidelines in 2013, and since then, new drugs and devices have been developed. This review presents an evidence-based approach to current heart failure management.
KEY POINTS
- Most patients with systolic heart failure (also called heart failure with reduced ejection fraction) should receive either an angiotensin-converting enzyme inhibitor or an angiotensin II receptor blocker. Most should also receive a beta-blocker (carvedilol, metoprolol succinate, or bisoprolol).
- If symptoms persist or progress despite these treatments, an aldosterone receptor antagonist (spironolactone or eplerenone) is recommended.
- Since the publication of the ACC/AHA guidelines in 2013, the combination of sacubitril and valsartan has been approved, as has ivabradine.
- Patients with advanced heart failure should be identified early for consideration of resynchronization therapy, an implantable cardiac defibrillator, digoxin, a left ventricular assist device, or heart transplant.
- B-type natriuretic peptide levels can be used to guide therapy.
Managing heart failure is a challenge. To aid clinicians in this task, the American College of Cardiology Foundation (ACC) and the American Heart Association (AHA) publish evidence-based guidelines, most recently in 2013.1 Since then, new drugs and devices have been shown to improve survival and reduce hospitalizations.
This paper reviews the ABCs of outpatient management of systolic heart failure (or heart failure with reduced ejection fraction), including the results of major trials and recommendations.

A common and serious condition
Heart failure is a debilitating syndrome that takes a significant physical and mental toll on those affected.
And it is common. An American age 40 or older faces a 20% lifetime risk of heart failure.1 An estimated 5.1 million Americans have clinical signs and symptoms of heart failure, and 900,000 new cases are diagnosed each year.2 By 2030 the prevalence of heart failure is projected to increase by 46%, and 9 million Americans will have been diagnosed with it.2
The severity of heart failure can be described using either the functional classification devised by the New York Heart Association (NYHA; Table 1) or the stages defined by the ACC and AHA.1,3 Though survival rates have improved, there is a direct correlation between worsening symptoms and death.4
Heart failure is the leading cause of hospitalizations annually. It accounts for $30 billion in healthcare costs, with direct medical costs accounting for 68% and another $1.8 billion associated with clinic visits, most often with primary care providers. By 2030, the cost is projected to increase by 127% to $69.7 billion—$244 per person in the United States.2
ACE inhibitors
The renin-angiotensin-aldosterone system has been studied for over 100 years.5
In heart failure with reduced ejection fraction, this system is upregulated as an adaptive mechanism to maintain hemodynamic homeostasis.6–8 However, prolonged activation of the renin-angiotensin-aldosterone system can lead to deleterious cardiovascular effects such as myocyte hypertrophy, myocardial fibrosis, sodium conservation, and fluid overload.8,9 Angiotensin II is a potent vasoconstrictor and plays a role in cardiovascular remodeling, leading to worsening progression of heart failure.6
CONSENSUS (the Cooperative North Scandinavian Enalapril Survival Study) examined the effect of the angiotensin-converting enzyme (ACE) inhibitor enalapril on survival in 253 patients with NYHA class IV heart failure. Participants were randomized to receive either enalapril or placebo. At 6 months, the mortality rate was 26% in the enalapril group vs 44% in the placebo group, an 18% absolute risk reduction and a 41% relative risk reduction (P = .002). At 12 months, the relative risk reduction in mortality was 30% (P = .001).10
SOLVD (the Study of Left Ventricular Dysfunction) extended the use of ACE inhibitors to all patients with heart failure, not just those in NYHA class IV. It randomized 1,284 patients with heart failure of any NYHA class and an ejection fraction less than 35% to receive either enalapril or placebo, and demonstrated a 16% relative risk reduction in mortality in the enalapril group, with mortality rates of 36% vs 39.7% (P = .0036).11
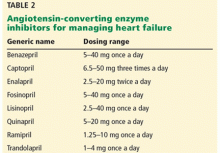
Recommendations. The benefits of ACE inhibition have been demonstrated in patients with mild, moderate, and severe heart failure. Thus, the guidelines recommend ACE inhibitors (Table 2) for all patients with heart failure with reduced ejection fraction.1
Angiotensin II receptor blockers
Angiotensin II receptor blockers (ARBs) (Table 3) have been proven to be suitable alternatives for patients with heart failure with reduced ejection fraction who cannot tolerate ACE inhibitors.
Val-HefT (the Valsartan HF Trial)12 randomized 5,010 patients in a double-blind fashion to receive either valsartan or placebo, with background therapy that included beta-blockers, digoxin, diuretics, and ACE inhibitors. There was a 13% reduction of the combined primary end point of mortality and morbidity and a 24% reduction in heart failure hospitalizations in the valsartan group.12
Subgroup analysis compared patients on the basis of use of ACE inhibitors and beta-blockers at study entry. Valsartan had a favorable effect in the subgroups using beta-blockers alone, ACE inhibitors alone, and neither drug. However, when patients received all three (a beta-blocker, an ACE inhibitor, and valsartan), the mortality rate was significantly increased (P = .009).12 This finding conflicted with those of other studies, which found a small benefit of combining an ACE inhibitor and an ARB.
CHARM-Added (the Candesartan in HF Assessment of Reduction in Mortality and Morbidity trial)13 investigated whether adding the ARB candesartan to an ACE inhibitor would improve clinical outcomes. In the study, 2,548 patients in NYHA class II, III, or IV with a left ventricular ejection fraction of less than 40% who were receiving ACE inhibitors were randomized to either candesartan or placebo. The addition of candesartan resulted in a significant reduction in cardiovascular mortality and heart failure hospitalizations, but with the downside of higher rates of hyperkalemia and serum creatinine elevation.13
Recommendations. The 2013 guidelines recommend that ARBs be used in patients who cannot tolerate an ACE inhibitor due to cough. However, routine combined use of ARBs, ACE inhibitors, and aldosterone antagonists is not recommended and may cause harm.1
Aldosterone receptor antagonists
Elevated levels of aldosterone lead to fluid retention, loss of magnesium and potassium, and myocardial fibrosis.
RALES (the Randomized Aldactone Evaluation Study)14 tested the hypothesis that the aldosterone receptor antagonist spironolactone (25 mg daily) would reduce deaths from all causes in patients with severe heart failure receiving standard medications including an ACE inhibitor. RALES included 1,663 patients in NYHA class III or IV with a left ventricular ejection fraction of 35% or less, randomized to receive 25 mg of spironolactone or matching placebo. This study found a 30% relative risk reduction and an 11% absolute risk reduction in all-cause mortality, a 31% relative risk reduction and a 10% absolute risk reduction in cardiac mortality, and 30% fewer cardiac-related hospitalizations in the spironolactone group.14
Eplerenone, an aldosterone receptor antagonist that lacks the antiandrogenic side effects of spironolactone, has also been shown to be beneficial. Its efficacy in patients with left ventricular systolic dysfunction was first established in postmyocardial infarction patients.15
EMPHASIS-HF (the Eplerenone in Mild Patients Hospitalized and Survival Study in Heart Failure)16 broadened the application of eplerenone (and aldosterone antagonists in general), investigating the effects of eplerenone in 2,737 NYHA class II patients, regardless of ischemic etiology. The composite end point of cardiovascular death or heart failure hospitalization occurred in 18.3% of the eplerenone group vs 25.9% of the placebo group (P < .001). A total of 12.5% of patients in the eplerenone group died, compared with 15.5% in the placebo group (P = .008). Hospitalizations were also fewer in the eplerenone group.
Recommendations. The 2013 guidelines recommend aldosterone receptor antagonists (Table 4) for patients with NYHA class II, III, or IV heart failure who have an ejection fraction of 35% or less, to reduce morbidity and mortality (class IA recommendation).1 The guidelines also recommend that these agents not be used in patients with renal insufficiency (serum creatinine > 2.5 mg/dL in men or > 2.0 mg/dL in women; an estimated glomerular filtration rate < 30 mL/min/1.73 m2); or a serum potassium level above 5 mmol/L.1
Angiotensin-neprilysin inhibitor (the future)
Research has identified neprilysin as another potential target in the treatment of heart failure and has sought to combine inhibition of angiotensin and neprilysin.
Neprilysin, a neutral endopeptidase, is associated with degradation of several natural vasoactive peptides such as natriuretic peptide, bradykinin, and adrenomedullin. Neprilysin inhibition increases these substances and counters the neurohormonal overactivation that leads to vasoconstriction, sodium retention, and cardiac remodeling.17
The ARB valsartan has been combined with the neprilysin inhibitor sacubitril to create the first angiotensin-neprilysin inhibitor (ARNI) (Table 5). The combination was selected to minimize the potential for angioedema.
PARADIGM-HF (the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trial)17 examined whether combined angiotensin-neprilysin inhibition was superior to ACE inhibition alone with enalapril in patients with chronic heart failure.
In PARADIGM-HF, 10,521 patients with NYHA class II, III, or IV heart failure were randomized to receive either sacubitril-valsartan or enalapril. The group receiving sacubitril-valsartan had significantly fewer deaths from cardiovascular causes and heart failure hospitalizations.17 An improvement in quality of life and NYHA functional class was also observed in the sacubitril-valsartan group.17
Sacubitril-valsartan underwent priority review by the US Food and Drug Administration and has been approved. Currently, it is indicated for the treatment of heart failure with reduced ejection fraction and NYHA class II, III, or IV symptoms. It should be avoided in patients who have previously experienced angioedema with an ACE inhibitor or ARB, in patients receiving aliskiren for diabetes, and in patients with hypersensitivity reactions to either of its components. Simultaneous use of sacubitril-valsartan and an ACE inhibitor should be avoided, and a washout period is recommended when transitioning from an ACE inhibitor to this combined agent.