Radiation-induced heart disease: A practical guide to diagnosis and management
ABSTRACTRadiation-induced heart disease (RIHD) is a recognized late sequela of chest radiotherapy for conditions such as breast cancer and lymphoma and can involve any cardiac structure. Consensus guidelines from the European Association of Cardiovascular Imaging and the American Society of Echocardiography stress the importance of regular screening for the cardiac effects of radiotherapy. However, a gulf remains between these guidelines and clinical practice.
KEY POINTS
- Ischemic heart disease is the most common cause of cardiac death after radiotherapy. Valvular, pericardial, myocardial, and conduction system disease are also common.
- Surgery may not be an attractive option because of radiation-induced fibrosis of surrounding structures. Consequently, conservative interventions are preferred.
- The incidence of RIHD is expected to decline, as lower doses of radiation are being used in radiotherapy than in the past.
PERICARDIAL DISEASE
Pericardial disease is a frequent manifestation of RIHD and covers a spectrum of manifestations from acute pericarditis, pericardial effusion, and tamponade to constrictive pericarditis. In a necropsy study, 70% of patients with RIHD were found to have pericardial involvement.32
The mechanism is believed to be radiation-induced microvascular injury resulting in increased capillary permeability and the sometimes rapid development of a protein-rich exudate. Associated inflammation may cause acute pericarditis, which may eventually be complicated by chronic pericarditis. The parietal surface tends to be affected more severely than the epicardium.33
Perhaps as a result of recent advances such as lower radiation doses, equal weighting of the anterior and posterior fields, and subcarinal blocking, incidence rates of pericarditis as low as 2.5% have been reported.34
Pericardial RIHD may be divided into early acute pericarditis, delayed chronic pericardial effusion, and constrictive pericarditis.
Early acute pericarditis is rare and is thought to represent a reaction to tumor necrosis. It is defined as occurring during radiotherapy and occurs almost exclusively with high-dose radiotherapy for lymphoma. Due to the relatively benign course of acute pericarditis and fear of tumor recurrence, it is not an indication to withhold radiotherapy.35
Delayed chronic pericardial effusion occurs months to years after radiotherapy, is typically asymptomatic, and presents as an enlarged cardiac silhouette on chest imaging.35 Delayed pericardial effusion is followed with imaging. While in many cases it resolves within 2 years, it may also be long-standing. Pericardiocentesis or a pericardial window may be performed to treat symptomatic effusion or delayed effusion causing hemodynamic compromise.35–37 Hypothyroidism should be ruled out, as it can complicate mantle irradiation and result in chronic pericardial effusion.38
Constrictive pericarditis may occur as a late complication of radiotherapy and typically causes symptoms of congestive heart failure. Pericardial stripping in these patients is complicated by the possibility of coexisting RIHD of the valves, myocardium, or coronary arteries, as well as mediastinal fibrosis. A study of 163 patients who underwent pericardial stripping for chronic pericarditis found a 7-year overall survival rate of only 27%, far lower than the rate for those who had no history of radiation exposure.39 Therefore, these patients are often treated for symptom control with diuretics and a low-salt diet rather than with surgery.
MYOCARDIAL DISEASE
Microvascular injury in the myocardium results in chronic ischemia, which may lead to myocardial fibrosis, typically manifesting as diastolic dysfunction. Chest radiotherapy may result in both systolic and diastolic dysfunction, and dilated and restrictive cardiomyopathy are well-recognized complications.40
Historically, high radiation doses resulted in systolic dysfunction in more than half of patients who underwent thoracic radiotherapy.41 Now, however, fewer than 5% of patients develop reductions in left ventricular ejection fraction, and most cases of radiotherapy-induced cardiomyopathy have a restrictive pattern.42
In a single-institution study, diastolic dysfunction was reported in as many as 14% of patients who underwent thoracic radiotherapy for Hodgkin lymphoma.40 Systolic dysfunction is now seen almost exclusively in patients treated concurrently with cardiotoxic chemotherapeutic agents such as anthracyclines in addition to radiotherapy.43
In a childhood cancer survival series, the hazard ratio of congestive heart failure in patients who had undergone radiotherapy for Wilms tumor was 6.6—almost identical to the occurrence in sibling controls. By contrast, the hazard ratio increased to 18.3 in those who received doxorubicin in addition to radiotherapy.44
Treatment of radiation-induced cardiomyopathy
Treatment of radiation-induced cardiomyopathy is similar to that for other forms of cardiomyopathy, with an emphasis on symptom management.
Heart transplant may be an option for highly selected patients with end-stage heart failure secondary to RIHD. In one report, a series of four RIHD patients received a heart transplant, and all four survived past 48 months.45 However, data from the United Network of Organ Sharing revealed an increase in the all-cause mortality rate in patients undergoing heart transplant for RIHD compared with those undergoing transplant for cardiomyopathy due to other causes.46 This trend may be confounded by a higher prevalence of prior cardiac surgery in the RIHD group—itself an established risk factor for poor posttransplant outcomes.
CONDUCTION SYSTEM DISEASE
Life-threatening arrhythmias have been reported that are distinct from the common, asymptomatic repolarization abnormalities that occur during radiotherapy. Atrioventricular nodal bradycardia, all degrees of heart block, and sick sinus syndrome have all been reported after chest radiotherapy. As conduction abnormalities do not typically manifest until years after radiotherapy, it is difficult to establish causation and, consequently, to define incidence.
Right bundle branch block is the most common conduction abnormality because of the proximity of the right bundle to the endocardium on the right side.47
Chest radiotherapy is also associated with prolongation of the corrected QT interval (QTc). A study in patients with a history of thoracic radiotherapy found that the QTc characteristically increased with exercise, a poor prognostic indicator.48 In a study of 134 survivors of childhood cancer, 12.5% of those who had undergone radiotherapy had a resting QTc of 0.44 msec or more.49
Furthermore, a study of 69 breast cancer survivors found a higher incidence of conduction abnormalities at 6 months and 10 years after radiotherapy compared with baseline. The characteristic electrocardiographic changes at 6 months were T-wave changes. At 10 years, the T-wave abnormalities had resolved and were replaced by ST depression.50
As mentioned above, establishing radiotherapy as a cause for these conduction abnormalities is challenging, given the lag between radiation therapy and electrocardiographic changes. The following criteria have been proposed for establishing a link between atrioventricular blockade and prior radiation51:
- Total radiation dose to the heart > 40 Gy
- Delay of 10 years or more since therapy
- Abnormal interval electrocardiographic changes such as bundle branch block
- Prior pericardial involvement
- Associated cardiac or mediastinal lesions.
SCREENING GUIDELINES
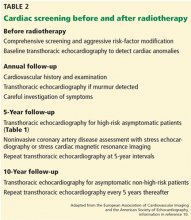
Consensus guidelines for identifying and monitoring RIHD have been published by the European Association of Cardiovascular Imaging and the American Society of Echocardiography (Table 2).10 The European Society of Medical Oncology has also issued guidelines for the prevention, diagnosis, and management of cardiovascular disease associated with cancer therapy.
Briefly, the guidelines call for aggressive cardiac risk-factor modification through weight loss, exercise, blood pressure control, and smoking cessation, in addition to early detection of RIHD. Cardiovascular screening for risk factors and a careful clinical examination should be performed in all patients. Baseline comprehensive transthoracic echocardiography is advocated in all patients before starting radiotherapy to detect cardiac anomalies. Beyond this, an annual history and physical examination, paying close attention to the signs and symptoms of cardiopulmonary disease, is essential. The development of new cardiopulmonary symptoms or a new physical finding such as a murmur should prompt evaluation with transthoracic echocardiography.
In patients without symptoms, screening transthoracic echocardiography at 10 years after the start of radiotherapy is recommended in light of the high probability of diagnosing cardiac disease at this juncture. In patients with no preexisting cardiac disease, surveillance transthoracic echocardiography should be at 5-year intervals thereafter.
In high-risk patients without symptoms (those who have undergone anterior or left-sided radiotherapy and have at least one risk factor for RIHD), initial screening transthoracic echocardiography is recommended 5 years after radiotherapy. These patients have a heightened risk of coronary events as described above and, consequently, are recommended to undergo noninvasive imaging 5 to 10 years after radiation exposure. If this initial examination is negative, stress testing should be repeated at 5-year intervals. Stress echocardiography and stress cardiac magnetic resonance imaging have higher specificity than stress electrocardiography and therefore are generally preferred. Stress scintigraphy should be used with caution, as it adds to the cumulative radiation exposure.
The role of magnetic resonance imaging and computed tomography depends on the results of initial transthoracic echocardiography and the clinical indication, in addition to the center’s expertise and facilities. However, there are currently no data advocating their use as screening tools, except for early detection of porcelain aorta in high-risk patients.10
MODERN RADIOTHERAPY TECHNIQUES
In recent years, there has been emphasis on exposing the patient to as little radiation as possible without compromising cure.52 The three major strategies employed to decrease cardiac exposure include reducing the radiation dose, reducing the radiation field and volume, and using newer planning and delivery techniques.
Reducing the radiation dose. It is well recognized that the mean dose of radiation to the heart is a significant predictor of cardiovascular disease, with one study demonstrating a linear increase in the risk of coronary artery disease with increasing mean heart radiation dose (excess relative risk per Gy 7.4%, 95% confidence interval 3.3%–14.8%).53
Reducing the radiation field and volume. Modern strategies and computed tomography-based radiotherapy planning have enabled a transition from older techniques such as extended-field radiation therapy, mantle-field radiation therapy, and involved-field radiation therapy to new techniques such as involved-node and involved-site radiation therapy.54 These have shown promise. For instance, a study in patients with early Hodgkin lymphoma found a mean heart dose of 27.5 Gy with mantle-field therapy compared with 7.7 Gy with involved-node therapy. This decrease in mean heart dose was associated with a reduction in the 25-year absolute excess cardiac risk from 9.1% to 1.4% and a reduction in cardiac mortality from 2.1% to 1%.55
Employing newer planning and delivery systems has also demonstrated some promise in reducing rates of cardiac morbidity and mortality. Extended-field radiation therapy, mantle-field radiotherapy, and involved-field radiation therapy were traditionally based on two-dimensional planning and often resulted in large volumes of myocardium being unnecessarily exposed to large doses of radiation because of the uncertainty in targeting. Involved-site and involved-node radiotherapy are based on computed tomography, resulting in more accurate targeting and sparing of normal tissue.
In addition, newer techniques such as intensity-modulated radiotherapy and proton beam therapy have resulted in further improvements in conformality compared with three-dimensional conformal radiotherapy.56,57 Respiratory motion management, including deep inspiration breath-holding and end-inspiration breath-holding, have decreased the radiation dose to the heart in patients undergoing mediastinal radiotherapy.58,59
TOWARD THE GOALS OF PREVENTION AND EARLIER DETECTION
As survival from breast cancer and lymphoma has increased, we continue to see legacy or latent effects of therapy, such as RIHD. Radiation therapy can affect any cardiac structure and is a major cause of morbidity and death in cancer survivors.
Modern radiation techniques use a variety of mechanisms to decrease the radiation dose to the heart. A large body of evidence emanating from an era of higher radiation doses and a lack of knowledge of the cardiac effects of radiation highlight the perilous cardiac consequences of chest radiation. With advances in radiotherapy and the development and widespread implementation of consensus guidelines, we envision earlier detection and less frequent occurrence of RIHD, although the latter trend could be blunted by increased cardiovascular risk factors within the population. Given the lag between irradiation and the cardiac consequences, it may be a number of years before any comparisons can be drawn.