Bioresorbable stents: The future of interventional cardiology?
ABSTRACTThe introduction of stents has drastically reduced target-lesion restenosis rates associated with percutaneous coronary angioplasty. Bare-metal stents were the first introduced, followed by drug-eluting stents, both of which had significant impacts on the complication rates. Stents, however, have resulted in the emergence of stent thrombosis and stent restenosis, which can cause life-threatening cardiac complications. Three new technological approaches are being investigated to overcome these complications: stents coated with bioresorbable polymers, stents without polymers, and completely bioresorbable stents. Initial results are encouraging, but more data are needed to ascertain their implications for clinical practice.
KEY POINTS
- Stents have dramatically improved outcomes associated with percutaneous coronary angioplasty.
- Bare-metal stents were the first stents developed, followed by first- and second-generation drug-eluting stents, which have progressively reduced complication rates.
- Despite the improvements with conventional stents, persistent rates of restenosis and stent thrombosis remain, which can lead to increased coronary morbidity and mortality.
- New stent technologies include stents coated with bioresorbable polymers, stents without polymers, and completely bioresorbable stents.
CAN WE SOLVE THE PROBLEM?
Three technological approaches have been proposed to overcome stent thrombosis and restenosis:
- Stents coated with bioresorbable polymers that quickly degrade
- Stents without polymers
- Stents that are completely resorbed.
STENTS WITH BIORESORBABLE POLYMERS
,As described above, the presence of a polymer on the stent predisposes it to inflammation. Therefore, it would be logical to hypothesize that a bioresorbable polymer would reduce the inflammation. This approach is typified by the second-generation paclitaxel-eluting stent (Synergy, Boston Scientific). It has a biodegradable coating that resorbs within 4 months and releases everolimus in a dose intensity similar to that seen with the contemporary second-generation DES.
The largest trial of this device to date, the Evolve II study, randomly assigned 1,684 patients to the biostable-polymer, everolimus-eluting chromium stent (Promus, Boston Scientific) or the paclitaxel-eluting stent (Synergy, Boston Scientific).10 Two-year follow-up data suggest that the rate of target-lesion failure was 9.4% in the paclitaxel-eluting stent patients vs 8.5% in the everolimus-eluting stent patients. Notably, no definite stent thrombosis was seen in the Synergy-treated patients 24 hours after the initial device implantation.
STENTS WITHOUT POLYMERS
If polymers predispose to inflammation, stents without polymers should mitigate the risk. Such stent types are exemplified by the BioFreedom (Biosensors International) stainless steel stent, a polymer-free umirolimus (also known as biolimus A9)-eluting stent. These stents have a microstructured surface that holds the drug without a polymer and releases the active drug over a few months.
The LEADERS FREE clinical trial studied this stent in 2,466 patients at high risk of bleeding.11 The patients were randomized to receive either a BMS or the polymer-free stent. All patients were required to receive dual antiplatelet therapy for only 1 month. At 1 year, the composite risk of cardiac death, myocardial infarction, and stent thrombois was 9.4% in patients with BioFreedom stents vs 12.9% in BMS patients. Of note, the primary end point did not include stent restenosis, thereby not disadvantaging the BMS.
Medtronic’s polymer-free, sirolimus-eluting stent is currently under investigation in the RevElution clinical trial.12 It has a cylindrical structure with the core replaced by the active drug sirolimus. Abluminal holes in the stent allow controlled release of the drug. A pharmacokinetic analysis show that 90% of the medication is released within the first 90 days and that tissue concentrations are maintained in the therapeutic range until at least that time.13 This actually exceeds that of the second-generation everolimus-eluting DES.
BIORESORBABLE STENTS
Bioresorbable scaffolds or stents disappear entirely over time and have drawn considerable attention in the interventional cardiology community. The FDA recently approved Abbott’s Poly-L-Lactic Acid (PLLA) everolimus-eluting stent (Absorb). The rate of bioresorption of this device can be controlled by modulating the respective contribution of amorphous and crystalline PLLA backbone. The advantage of bioresorbable stents appears to stem from the fact that with bioresorbable devices, the vessel may actually expand and the purported nidus for inflammation goes away. This has been demonstrated by serial intravascular ultrasound-based studies.14
The return of pulsatility also appears to modulate the transition of smooth muscles from proliferative back to their contractile phenotype. This has been hypothesized to reduce the risk of neoatherosclerosis and, consequently, stent restenosis. The limitation of this device is the large strut size (157 micron for Absorb vs 81 microns for Xience). Dissolving metallic scaffolds also tend to have thicker struts than the current DES (120 vs approximately 80 microns).
The Absorb III trial was a pivotal noninferiority US trial that led to the device approval.15 In this trial, 2,008 patients were randomized to receive the Absorb bioresorbable, everolimus-eluting stent or the DES Xience. The primary study end point was target-lesion failure at 1 year. As is often the case with US landmark studies, patient and lesion complexities were limited. Patients with acute coronary syndrome, elevated cardiac enzymes, high-risk anatomic lesions such as bifurcation lesions, and chronic total occlusion were excluded. Patients with diabetes comprised less than one-third of the patients, and lesions were relatively short at 13 ± 6 mm.
Device success per lesion was lower with Absorb than with Xience (94.3% vs 99.3%; P < .0001). This is likely due to the larger strut size. Absorb III did meet the prespecified primary end point for noninferiority (P = .007), although the rate of adverse events was somewhat higher (7.8% vs 6.1%). A subgroup analysis reveals that 19% of all lesions were smaller than what was originally intended, and in these patients, the Absorb device performed poorly with a 4.6% risk of device thrombosis. When limited to patients with the intended reference vessel sizes, the results of target-lesion failure and stent thrombosis were similar (6.6% vs 5.5% and 0.8% vs 0.5%, respectively).15
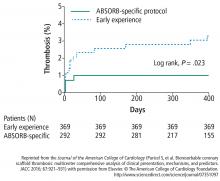
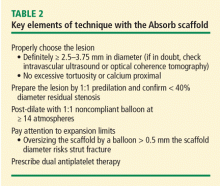
The implantation technique also seems to have influenced the results, with increased use of post-dilation as the study evolved. Recent observations from the MICAT group have shown that the use of high pressure post-dilation and other procedural advancements may considerably reduce adverse outcomes associated with Absorb (Figure 4).16 Thus, while the pooled analysis in the form of a meta-analysis has suggested an increased risk of device thrombosis,17 the difference is attenuated by selecting lesions of appropriate size, high-pressure post-dilation, and procedural advancements (Table 2).
CONCLUSION AND THE WAY FORWARD
Current first-generation bioresorbable stents can achieve results similar to those of second-generation DES, provided that they are used in patients with noncomplicated coronary lesions and the implant techniques are optimized. We do not know the outcomes of bioresorbable stents in patients with complex lesions. Current experience suggests that other changes in technique would be needed. For example, minimizing scaffold overlap in long and bifurcating lesions. Whether that would translate into diminishing the rate of late adverse events remains to be determined. As of now, we only have data on approximately 100 highly selected patients beyond 3 years (no adverse events 2.5 to 5 years after implantation).
Several investigational second-generation bioresorbable stents, including Elixir’s Dissolve PLLA, Boston Scientific’s FAST, and a newer version of Absorb, are in early clinical trials. Smaller strut thickness holds the promise of attenuating the risk of stent thrombosis. Since the polymer persists, no reduction in dual antiplatelet therapy duration is likely to be achieved.
Results from long-term follow-up of Absorb III and on-going trials are eagerly awaited to ascertain whether the rate of late complications of DES can be mitigated. It would not be surprising if the second-generation bioresorbable stents make DES a thing of the past within the next decade.