Transcatheter mitral valve replacement: A frontier in cardiac intervention
ABSTRACTAs transcatheter aortic valve replacement (TAVR) has become routine, device manufacturers and investigational cardiologists have set their sights on the mitral valve. Although transcatheter mitral valve replacement (TMVR) poses several technical challenges, they appear to be surmountable, and work is proceeding. Here we review the various devices being developed and preliminary results of trials in humans.
KEY POINTS
- Most TMVR procedures are performed by either a retrograde transapical approach or an antegrade transseptal approach.
- In the small number of patients who have undergone TMVR for native mitral valve regurgitation to date, mortality rates at 30 days have been high, reflecting the seriousness of illness in these patients.
- At present, none of the new devices for TMVR in patients with native mitral valve regurgitation are approved for general use, although some of them are being tested in phase 1 clinical trials that are enrolling patients.
- Valves made for TAVR have been used for TMVR in patients with degenerative mitral stenosis or failure of mitral bioprostheses; however, these are off-label uses of these devices.
In the last 10 years, we have seen a revolution in transcatheter therapies for structural heart disease. The most widely embraced, transcatheter aortic valve replacement (TAVR) was originally intended for patients in whom surgery was considered impossible, but it has now been established as an excellent alternative to surgical aortic valve replacement in patients at high or intermediate risk.1–3 As TAVR has become established, with well-designed devices and acceptable safety and efficacy, it has inspired operators and inventors to push the envelope of innovation to transcatheter mitral valve replacement (TMVR).
This review summarizes the newest data available for the TMVR devices currently being tested in patients with native mitral regurgitation, bioprosthetic degeneration, and degenerative mitral stenosis.
THE MITRAL VALVE: THE NEW FRONTIER
,Whereas the pathologic mechanisms of aortic stenosis generally all result in the same anatomic consequence (ie, calcification of the valve leaflets and commissures resulting in reduced mobility), mitral valve regurgitation is much more heterogeneous. Primary (degenerative) mitral regurgitation is caused by intrinsic valve pathology such as myxomatous degeneration, chordal detachment, fibroelastic deficiency, endocarditis, and other conditions that prevent the leaflets from coapting properly. In contrast, in secondary or functional mitral regurgitation, the leaflets are normal but do not coapt properly because of apical tethering to a dilated left ventricle, reduced closing forces with left ventricular dysfunction, or annular dilation as the result of either left ventricular or left atrial dilation.
Surgical mitral valve repair is safe and effective in patients with degenerative mitral regurgitation caused by leaflet prolapse and flail. However, some patients cannot undergo surgery because they have comorbid conditions that place them at extreme risk.4 For example, most patients with functional mitral regurgitation due to ischemic or dilated cardiomyopathy have significant surgical risk and multiple comorbidities, and in this group surgical repair has limited efficacy.5 A sizeable proportion of patients with mitral regurgitation may not be offered surgery because their risk is too high.6 Therefore, alternatives to the current surgical treatments have the potential to benefit a large number of patients.
Similarly, many patients with degenerative mitral stenosis caused by calcification of the mitral annulus also cannot undergo cardiac surgery because of prohibitively high risk. While rheumatic disease is the most common cause of mitral stenosis worldwide, degenerative mitral stenosis may be the cause in up to one-fourth of patients overall and up to 60% of patients older than 80 years.7 In the latter group, not only do old age and comorbidities such as diabetes mellitus and chronic kidney disease pose surgical risks, the technical challenge of surgically implanting a prosthetic mitral valve in the setting of a calcified annulus may be significant.8
The mitral valve is, therefore, the perfect new frontier for percutaneous valve replacement therapies, and TMVR is emerging as a potential option for patients with mitral regurgitation and degenerative mitral stenosis. The currently available percutaneous treatment options for mitral regurgitation include edge-to-edge leaflet repair, direct and indirect annuloplasty, spacers, and left ventricular remodeling devices (Table 1).9,10 As surgical mitral valve repair is strongly preferred over mitral valve replacement, the percutaneous procedures and the devices that are used are engineered to approximate the current standard surgical techniques. However, given the complex pathologies involved, surgical repair often requires the use of multiple repair techniques in the same patient. Therefore, percutaneous repair may also require more than one type of device in the same patient and may not be anatomically feasible in many patients. Replacing the entire valve may obviate some of these challenges.
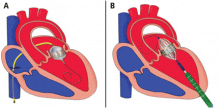
Compared with the aortic valve, the mitral valve poses a greater challenge to percutaneous treatment due to its structure and dynamic relationship with the left ventricle. Some specific challenges facing the development of TMVR are that the mitral valve is large, it is difficult to access, it is asymmetrical, it lacks an anatomically well-defined annulus to which to anchor the replacement valve, its geometry changes throughout the cardiac cycle, and placing a replacement valve in it entails the risk of left ventricular outflow tract obstruction. Despite these challenges, a number of devices are undergoing preclinical testing, a few are in phase 1 clinical trials, and registries are being kept. Depending on the specific device, an antegrade transseptal approach to the mitral valve (via the femoral vein) or a retrograde transapical approach (via direct left ventricular access) may be used (Figure 1).
NATIVE MITRAL VALVE REGURGITATION
For degenerative mitral regurgitation, the standard of care is cardiac surgery at a hospital experienced with mitral valve repair, and with very low rates of mortality and morbidity. For patients in whom the surgical risk is prohibitive, percutaneous edge-to-edge leaflet repair using the MitraClip (Abbott Vascular, Minneapolis, MN) is the best option if the anatomy permits. If the mitral valve pathology is not amenable to MitraClip repair, the patient may be evaluated for TMVR under a clinical trial protocol.
For functional mitral regurgitation, the decisions are more complex. If the patient has chronic atrial fibrillation, electrical cardioversion and antiarrhythmic drug therapy may restore and maintain sinus rhythm, though if the left atrium is large, sinus rhythm may not be possible. If the patient has left ventricular dysfunction, guideline-directed medical therapy should be optimized; this reduces the risk of exacerbations, hospitalizations, and death and may also reduce the degree of regurgitation. If the patient has severe left ventricular dysfunction and a wide QRS duration, cardiac resynchronization therapy (biventricular pacing) may also be beneficial and reduce functional mitral regurgitation. If symptoms and severe functional mitral regurgitation persist despite these measures and the patient’s surgical risk is deemed to be extreme, options include MitraClip placement as part of the randomized Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy (COAPT) trial, which compares guideline-directed medical therapy with guideline-directed therapy plus MitraClip. Another option is enrollment in a clinical trial or registry of TMVR.
At this writing, six TMVR devices have been implanted in humans:
- Fortis (Edwards Lifesciences, Irvine, CA)
- Tendyne (Tendyne Holding Inc, Roseville, MN)
- NaviGate (NaviGate Cardiac Structures, Inc, Lake Forest, CA)
- Intrepid (Medtronic, Minneapolis, MN)
- CardiAQ (Edwards Lifesciences, Irvine, CA)
- Tiara (Neovasc Inc, Richmond, BC).
Most of the early experience with these valves has not yet been published, but some data have been presented at national and international meetings.
The Fortis valve
The Fortis valve consists of a self-expanding nitinol frame and leaflets made of bovine pericardium and is implanted via a transapical approach.
The device was successfully implanted in three patients in Quebec City, Canada, and at 6 months, all had improved significantly in functional class and none had needed to be hospitalized.11 Echocardiographic assessment demonstrated trace or less mitral regurgitation and a mean transvalvular gradient less than 4 mm Hg in all.
Bapat and colleagues12 attempted to implant the device in 13 patients in Europe and Canada. The average left ventricular ejection fraction was 34%, and 12 of 13 patients (92%) had functional mitral regurgitation. Procedural success was achieved in 10 patients, but five patients died within 30 days. While the deaths were due to nonvalvular issues (multiorgan failure, septic shock, intestinal ischemia after failed valve implantation and conversion to open surgery, malnutrition leading to respiratory failure, and valve thrombosis), the trial is currently on hold as more data are collected and reviewed. Among the eight patients who survived the first month, all were still alive at 6 months, and echocardiography demonstrated no or trivial mitral regurgitation in six patients (80%) and mild regurgitation in two patients (20%); the average mitral gradient was 4 mm Hg, and there was no change in mean left ventricular ejection fraction.
The Tendyne valve
The Tendyne valve is a self-expanding prosthesis with porcine pericardial leaflets. It is delivered transapically and is held in place by a tether from the valve to the left ventricular apex.
In the first 12 patients enrolled in an early feasibility trial,13 the average left ventricular ejection fraction was 40%, and 11 of the 12 patients had functional mitral regurgitation. The device was successfully implanted in 11 patients, while one patient developed left ventricular outflow tract obstruction and the device was uneventfully removed. All patients were still alive at 30 days, and the 11 patients who still had a prosthetic valve did not have any residual mitral regurgitation.
As of this writing, almost 80 patients have received the device, though the data have not yet been presented. Patients are being enrolled in phase 1 trials.
The NaviGate valve
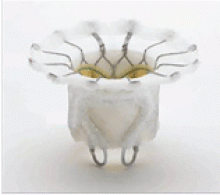
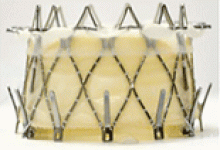
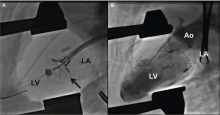
The NaviGate valve consists of a trileaflet subassembly fabricated from bovine pericardium, mounted on a self-expanding nitinol stent, and is only implanted transatrially.
NaviGate valves were successfully implanted in two patients via a transatrial approach (Figure 2). Both patients had excellent valve performance without residual mitral regurgitation or left ventricular outflow tract obstruction. The first patient showed significant improvement in functional class and freedom from hospitalization at 6 months, but the second patient died within a week of the implant due to advanced heart failure.14 A US clinical trial is expected soon.