Antihyperglycemic drugs and cardiovascular outcomes in type 2 diabetes
ABSTRACTIn patients with diabetes, a complex and controversial relationship exists between intensive glycemic control and cardiovascular (CV) outcomes. Although the value of glucose-lowering agents in preventing microvascular complications associated with diabetes has been established, along with reductions in ischemic coronary events, active treatment in one major glycemic-control trial resulted in an unexplained increase in CV-associated mortality and total deaths compared with controls. Questions of CV safety with specific glucose-lowering agents along with the mechanisms underlying their effects on CV events have not been fully answered, underscoring the need for additional well-designed, long-term randomized controlled trials (RCTs) to prove their CV safety vs an active comparator. The CV benefits of one sodium-glucose cotransporter-2 inhibitor reported in an RCT await confirmation in ongoing trials.
KEY POINTS
- Long-term randomized controlled trials have established the value of intensive glycemic control in reducing CV outcomes in patients with type 2 but only after many years of follow-up.
- Despite reductions in ischemic coronary events, some clinical trials have reported unexplained increases in CV-associated mortality and total deaths in patients receiving intensive glycemic control.
- Trials reporting the impact of specific glucose-lowering agents on CV events have reported perplexing, sometimes contradictory results, underscoring the need for additional trials.
Incretin-mimetic agents and CV outcomes
Following the FDA guidance,14 all newer agents, including incretin-mimetic agents (dipeptidyl peptidase-4 [DPP-4] inhibitors and glucagon-like peptide-1 [GLP-1] receptor agonists) and sodium-glucose cotransporter-2 (SGLT-2) inhibitors have been undergoing well-designed, long-term, noninferiority trials with the comparison group receiving the standard of diabetes and CV care. The goal of these trials, unlike that of most of the studies discussed in this article, was to investigate the safety of individual agents rather than different levels of glycemic control.
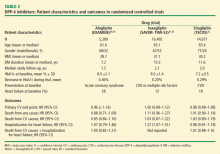
Since 2013, such trials with three of the available DPP-4 inhibitors have been completed (Table 2).30–34 Each trial was conducted in patients with pre-existing CV disease or high risk of it. The mean duration of follow-up in these trials was 1.5 to 3.0 years. There were significant, but only marginal, differences in HbA1c compared with the control groups receiving standard care. In each trial, the primary CV end points showed noninferiority, thus documenting their CV safety. One important difference in secondary end points was a significant increase in hospitalization rates for heart failure with saxagliptin31,33 that was not observed in the trials with alogliptin30,34 or sitagliptin.32 Another secondary end point—hospitalization for heart failure plus CV mortality—also was not increased in the alogliptin34 and sitagliptin32 trials (rates not reported for saxagliptin); however, there was no increase in total deaths from any cause in these trials.
,The mechanisms underlying the increased rates of heart failure with saxagliptin are unclear. The baseline characteristics of patients in these three trials were similar (Table 2). Patients with type 2 diabetes have higher rates of heart failure in general, but the effects of concomitant drug therapy on risk of heart failure, other than with TZDs, have not been well studied. In an extensive meta-analysis of 84 RTCs of various durations, the overall risk (OR) of heart failure was higher in patients treated with DPP-4 inhibitors than in those treated with placebo or active comparators (OR, 1.19; 95% CI, 1.03–1.37; P = .015), suggesting that DPP-4 inhibitors as a class could be associated with an increased risk of heart failure.35 A case-control study, however, found no increase in rates of heart failure with DPP-4 inhibitors, although there were very few patients on saxagliptin.36 Yet another large retrospective, propensity-adjusted observational analysis of more than 112,000 patients, which compared those on saxagliptin and sitagliptin, reported no difference in rates of heart failure; however, the median follow-up period was less than 6 months.37
In comparative observational analyses,18,37,38 the risks of heart failure with TZDs and sulfonylureas were increased, compared with DPP-4 inhibitors, particularly with TZDs. On the other hand, a large population-based analysis from Italy found that DPP-4 inhibitors were associated with a propensity-matched 36% lower rate of hospitalization for heart failure compared with sulfonylureas.39 These data point to a need for more well-designed comparative studies to investigate valid differences between drugs in this class.
In the only GLP-1 receptor agonist trial completed thus far, ELIXA (Evaluation of Lixisenatide in Acute Coronary Syndrome), there were no differences in primary and major secondary CV outcomes in 6,068 very high-risk patients randomized to lixisenatide or placebo after a 25-month follow-up (HR, 1.02; 95%, CI, 0.89–1.17).40 Moreover, the hospitalization rates for heart failure were not increased (HR, 0.96; 95% CI, 0.75–1.23). The earlier meta-analyses of short-term studies with DPP-4 inhibitors reporting significant reductions in CV events41,42 also underscore the need for well-designed long-term RCTs to accurately interpret drug effects.
SGLT-2 inhibitors and CV outcomes
The first CV outcome RCT with the SGLT-2 inhibitor empagliflozin, the EMPA-REG OUTCOME trial,43 was recently reported. Of great importance in this 7,020-patient trial comparing empagliflozin with placebo were the following results:
- 14% reduction in the primary end point (composite of death from CV causes, nonfatal MI, or nonfatal stroke) (P = .04)
- 32% reduction in all-cause deaths (P < .001)
- 35% reduction in hospitalization for heart failure (P = .002).
The mechanism underlying these impressive benefits is not known, although there were modest reductions in HbA1c levels, body weight (~2 kg), waist circumference (~2 cm), and systolic blood pressure (~4 mm Hg) with empagliflozin. The main adverse effects were related to a 3 to 4 times increased incidence of genital infections. Trials with other agents in this class are currently ongoing.
Insulin and CV outcomes
The UKPDS trial is the only primary prevention trial that provided evidence of significant benefits from intensive glucose control (with insulin, with or without sulfonylurea therapy) on CV outcomes and mortality, but only after 10 additional years of follow-up after the end of the trial.4 A few other trials have investigated the long-term effects of insulin compared with conventional therapy in patients with CV disease.
The DIGAMI-1 (Diabetes Insulin-Glucose in Acute Myocardial Infarction) was a RCT conducted between 1990 and 1993 in 620 patients with type 2 diabetes and acute MI randomized to short-term, intensive insulin-based glucose therapy or to conventional glucose-lowering therapy.44 Results showed the intensive treatment group had an 11% decrease in mortality rate at 3.4 years. A 20-year follow-up reassessment showed the overall survival was improved by a mean of 2.3 years at 8 years, particularly in those at lower risk at baseline.45 However, none of these patients were on statin therapy at baseline; thus, the implications of that study with current standards of care are quite uncertain. Subsequent studies—DIGAMI-2 (N = 1,253)46 and the HI-5 (Hyperglycemia: Intensive Insulin Infusion in Infarction) study (N = 240),47 both investigating the effects of intensive insulin therapy in patients with type 2 diabetes and MI—showed no significant effects on mortality in patients at 1 year (DIGAMI-2) and 6 months (HI-5).
The HEART2D trial (Hyperglycemia and Its Effect After Acute Myocardial Infarction on Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus), an RCT of 1,115 post-MI patients, investigated the effects of targeting prandial insulin compared with basal insulin. During a mean follow-up of 2.7 years, there were no between-group differences in CV outcomes (HR, 0.98; 95% CI, 0.8–1.21) or glycemic control.48 Also, there was no impact of glycemic variability.49 Finally, the ORIGIN trial (Outcome Reduction With an Initial Glargine Intervention), an RCT of more than 12,000 patients at high risk for CV disease but with relatively recent onset of either type 2 diabetes or prediabetes, randomized patients to basal insulin glargine or noninsulin treatments.50 The baseline HbA1c was relatively low at 6.4%, but it significantly declined by 0.3% by the end of trial, compared with the control group. There was no effect on CV outcomes (HR, 1.02; 95% CI, 0.94–1.11) after a median follow-up of 6.2 years.
However, it remains a perplexing question regarding whether long-term treatment with increasing insulin dosages in a subset of obese patients with poorly controlled type 2 diabetes and increasing insulin resistance could be potentially harmful to the CV system.51
CONCLUSION
The long-term RCTs with antihyperglycemic agents, including DCCT/EDIC in type 1 diabetes and UKPDS, ACCORD, and VADT in type 2 diabetes, with the exception of ADVANCE, have established the value of intensive glycemic control in reducing CV outcomes but only after many years of follow-up. However, the effects of intensive glycemic control on CV disease in type 2 diabetes are inconsistent, with only the primary prevention cohorts of UKPDS showing significant effects on mortality after prolonged follow-up. This is in contrast to the positive effects of statins in relatively short-term trials.
While it is difficult to interpret the CV results of specific drugs from the degree of glycemic control, it is reassuring that the large RCTs with several individual agents, including TZDs (both pioglitazone and rosiglitazone), several DPP-4 inhibitors, and one GLP-1 receptor agonist, have demonstrated no appreciable harm. The increase in the secondary outcome of heart failure but with no increase in mortality observed with saxagliptin requires further mechanistic studies while awaiting the results of other ongoing trials with newer agents including other incretin-based drugs and SGLT-2 inhibitors.
With SGLT-2 inhibitors, the recently published results of the empagliflozin trail (EMPA-REG OUTCOME trial) with type 2 diabetes revealed a significant reduction in CV end points and mortality. Before those data were published, metformin was the only antihyperglycemic drug that had shown a significant effect on CV events and mortality, but it was studied in only a small subgroup of the UKPDS cohort, and there are no RCTs of the relative impact of metformin or other agents as compared to sulfonylureas. The results of ongoing CV trials with SGLT-2 inhibitors are eagerly awaited.