Newer oral and noninsulin therapies to treat type 2 diabetes mellitus
ABSTRACTThe pathophysiology of type 2 diabetes mellitus involves several biologic mechanisms and no single medication addresses them all. Most patients require more than one medication to adequately treat their diabetes, needing drugs with unique and complementary mechanisms of action to address and balance insulin and glucagon levels. In the past decade, several therapeutic drug classes have been developed for type 2 diabetes mellitus. Each provides therapeutic options with novel mechanisms of action to help clinicians achieve the goal of glucose homeostasis while controlling adverse events, especially reducing the risk of hypoglycemia.
KEY POINTS
- The US Food and Drug Administration has approved 14 noninsulin pharmaceuticals in five drug classes in the past decade for type 2 diabetes therapy.
- The noninsulin drug classes of dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, bile acid sequestrants, and dopamine-receptor agonists have different mechanisms of action and therapeutic effects.
- Successful management strategies require a balancing of multiple agents to achieve target glucose while avoiding adverse effects.
Type 2 diabetes mellitus (DM) is caused by hyperglycemia and metabolic alterations due to abnormalities in insulin secretion or insulin action, or both. To achieve desired glycemic targets, different antihyperglycemic drugs are used alone or in combination with other agents, including insulin. First-line options for diabetes treatment are weight loss, lifestyle modification, and metformin. The American Diabetes Association and the European Association for the Study of Diabetes recommend a patient-specific treatment approach to enhance glycemic control while avoiding weight gain and hypoglycemia.1 This review will focus on the newer oral agents and injectable noninsulin agents that are used to achieve glycemic control. Table 1 lists the noninsulin drugs approved since 2005.
INCRETIN-BASED THERAPIES
The incretins are glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), which are secreted by the gastrointestinal (GI) tract in response to food intake. Both GLP-1 and GIP stimulate beta cells of the pancreas, which contribute 60% of the insulin secretion after a meal. Type 2 DM is associated with decreased secretion of GLP-1 and lowered responsiveness to GIP. Benefits of the incretin hormones on glycemic control include enhanced satiety, decreased GI motility, increased glucose-dependent insulin secretion, reduced glucagon secretion, and decreased hepatic glucose release.2 Two incretin-based drug classes are used to treat patients with type 2 DM—oral dipeptidyl peptidase-4 (DPP-4) inhibitors and GLP-1 receptor agonists.
DPP-4 inhibitors
The oral DPP-4 inhibitors block the degradation of the enzyme DPP-4 active site and thus increase the GLP-1 and GIP concentrations by two to three times. Their primary effectiveness centers on controlling insulin and glucagon secretion without increasing weight.
Four DPP-4 inhibitors are approved by the US Food and Drug Administration (FDA) in once-daily oral formulations: sitagliptin, saxagliptin, linagliptin, and alogliptin. Another DPP-4 inhibitor, vildagliptin, is not licensed in the United States but is approved for use in Europe and Japan.3 Another DPP-4 inhibitor, teneligliptin, is also marketed in Japan.
The DPP-4 inhibitors are indicated for use as monotherapy or in combination with other agents such as metformin, sulfonylureas, thiazolidinediones, and insulin. Generally, DPP-4 inhibitors do not cause hypoglycemia when used as monotherapy.1 When adding a DPP-4 inhibitor to a sulfonylurea or insulin, it is recommended to decrease the sulfonylurea or insulin dose to reduce the risk of hypoglycemia. The potential of these agents to lower the hemoglobin A1c (HbA1c) when used as monotherapy and in combination with metformin, sulfonylureas, or thiazolidinediones is 0.3% to 0.71%.4
The DPP-4 inhibitors are not known to cause adverse GI effects. Sitagliptin, alogliptin, vildagliptin, and saxagliptin need dosing adjustments for renal insufficiency; however, linagliptin is not renally eliminated and does not require dosing adjustment. Common adverse events (> 5%) are nasopharyngitis, upper-respiratory infection, and headache. Sitagliptin and saxagliptin have been associated with urinary tract infection. Sitagliptin also has been associated with more extremity pain, back pain, and osteoarthritis.4
The DPP-4 inhibitors are primarily excreted by the renal or fecal route and, therefore, have few drug interactions. All DPP-4 inhibitors are partially metabolized through cytochrome P450 enzymes, except saxagliptin.4 Their pharmacokinetic profiles are shown on Table 2.
In keeping with the FDA guidelines, sitagliptin, saxagliptin, linagliptin, and alogliptin have been evaluated for cardiovascular (CV) outcomes. The SAVOR-TIMI 53 clinical trial (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients With Diabetes Mellitus–Thrombolysis in Myocardial Infarction 53) was a 2-year CV safety and efficacy trial.5 This trial demonstrated no statistically significant difference in the primary end point as a composite of CV death, myocardial infarction (MI), and ischemic stroke. Additionally, the secondary end points, including hospitalization for unstable angina, coronary revascularization, and heart failure, also did not show significant difference. However, based on a subgroup analysis, there was a statistically significant increase in patients in the saxagliptin group vs the placebo group who were hospitalized for heart failure.
A 2-year trial comparing linagliptin with glimepiride, a second-generation sulfonylurea, showed significantly fewer CV events with linagliptin.6 This trial found a relative risk reduction of 54% in the end points of CV death, nonfatal MI or stroke, and unstable angina during hospitalization.4,6 Another trial reviewed the incidence of CV events (CV death, nonfatal MI, and nonfatal stroke) in patients treated with alogliptin, placebo, or comparator antihyperglycemic drugs and found no increased incidence of major adverse CV events vs comparator therapies.7
The recently published TECOS (Trial Evaluating Cardiovascular Outcomes With Sitagliptin), reported no increase in major atherosclerotic CV events, no difference in all-cause mortality, and no difference in heart failure for hospitalization or other adverse events in patients with type 2 DM.8 Other clinical trials such as the CAROLINA study (linagliptin compared with glimepiride),9 the EXAMINE study (alogliptin),10 and the CARMALINA study (linagliptin)11 are also reviewing the CV safety of DPP-4 inhibitors in the United States.
Concern has been raised about the association between incretin-based therapies and adverse pancreatic effects. CV outcomes trials using saxagliptin and alogliptin found similar rates of pancreatitis and fewer pancreatic cancer cases in comparison with placebo.5,7,10 TECOS demonstrated that with sitagliptin, acute pancreatitis occurred more in the sitagliptin group, but there was no statistical significance reported. However, pancreatic cancer occurred more in the placebo group, although the difference was not statistically significant.8 Neither the FDA nor the European Medicines Agency (EMA) has reached a firm conclusion about the possible association between incretin-based therapies and pancreatitis or pancreatic cancer.12
GLP-1 agonists
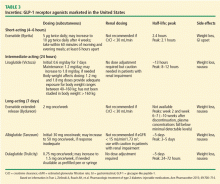
The GLP-1 drugs mimic the action of native GLP-1. Several GLP-1 agents are available in the United States, and several more are in development.11,13 The drug class is divided into three groups:
- Short-acting (4–6 hours): exenatide, lixisenatide
- Intermediate-acting (24 hours): liraglutide
- Long-acting (7 days): exenatide extended-release (ER), dulaglutide, and albiglutide (semaglutide is in phase 3 study).
The GLP-1 receptor agonists heighten glucose homeostasis by the following mechanisms of action: stimulate insulin secretion, suppress glucagon secretion, directly and indirectly inhibit endogenous glucose production, promote satiety, heighten insulin sensitivity due to weight loss, and slow gastric emptying time. Table 3 lists dosing and pharmacokinetic profiles for GLP-1 agonists. When GLP-1 agonists are used as monotherapy, the HbA1c is reduced by 0.7% to 1.51%.13 When GLP-1 agonists are used in combination with metformin, sulfonylureas, thiazolidinediones, or as three-drug therapy with other oral antidiabetic medications, the HbA1c is lowered by 0.4% to 1.9%.13–17
A notable advantage of GLP-1 agonists is their effect on weight loss separate from GI side effects. Weight reductions of 0.2 to 3.6 kg in 26 weeks have been seen with the exenatide formulations, liraglutide, albiglutide, and dulaglutide.13,18 Liraglutide has demonstrated a greater weight reduction than exenatide, exenatide ER, or albiglutide.13,16,17 There were similar weight reductions of 1.5 kg in 26 weeks in a comparator trial involving liraglutide and dulaglutide (3.6 vs 2.9 kg).19
Common adverse effects of the GLP-1 agonists are nausea (8% to 44%), diarrhea (6% to 20%), and vomiting (4% to 18%), which may occur initially and diminish with continued use.13,14 There have been more GI side effects with liraglutide than with exenatide ER or albiglutide.20 Increased rates of injection site reactions, such as transient small nodule formations, were seen with exenatide ER (5.4% to 17.6%) and albiglutide, the once-weekly GLP-1 agonist therapies, vs exenatide, liraglutide, and insulin glargine.13,14 Dulaglutide, another once-weekly GLP-1 agonist, does not have this finding; however, there is enhanced patient satisfaction with the once-weekly preparations in comparison with the twice-daily preparations.14,16 Patients who received albiglutide have noted hypersensitivity reactions such as pruritus, rash, and dyspnea (10% to 18%).13 Hypoglycemia is not seen with the GLP-1 agonists, unless they are used in conjunction with a sulfonylurea or insulin.
Exenatide and exenatide ER are excreted by the renal route; therefore, it is not recommended to use these agonists in patients with renal impairment or end-stage renal disease (creatinine clearance [CrCl] < 30 mL/min). Liraglutide is not excreted by the renal route; however, it should be used with caution in patients with renal impairment.21 No renal dose adjustment is required when using albiglutide or dulaglutide.21
Clinical trials have demonstrated the short-term CV outcomes of GLP-1 agonists. The CV benefits include a decrease in blood pressure, reduction of lipid levels, enhanced endothelial function, and improved myocardial function.13 One meta-analysis reported a tendency for lowering the rate of major CV events, stroke, MI, CV mortality, and all-cause mortality.22 Several ongoing trials are evaluating the safety of GLP-1 agonists and CV safety: LEADER (liraglutide), EXSCEL (exenatide LR), ELIXA (lixisenatide), SUSTAIN 6 (semaglutide), and REWIND (dulaglutide).11
The GLP-1 agonists have been linked to an increased incidence of thyroid cancer. There was a potential increased risk of thyroid cancer in preclinical rodent studies involving liraglutide and exenatide ER, but this risk was not demonstrated for the exenatide twice-daily preparation.13 The FDA noted that the findings from rodent studies, which demonstrated a possible heightened risk for thyroid cancer, should not be conveyed to the outcomes for humans. Nevertheless, when liraglutide was approved in January 2010, the FDA issued a boxed warning about the risk of thyroid C-cell hyperplasia. The package inserts list a thyroid carcinoma risk for exenatide ER, liraglutide, albiglutide, and dulaglutide in those patients with a personal or family history of medullary thyroid cancer.
There is controversy about the incidence of pancreatitis and pancreatic cancer with the use of the incretin-based therapies. Published studies and case reports seem to support speculation that there is an increased incidence of acute pancreatitis associated with type 2 DM.21 The FDA and the EMA have independently reviewed postmarketing reports about pancreatitis and pancreatic cancer among more than 28,000 patients who received some form of incretin-based therapy.12 They independently agreed that a causal association between incretin-based drugs and pancreatitis or pancreatic cancer is inconsistent with the current data.12 At this time, there is no final conclusion about a causal relationship between the use of incretin-based drugs and possible pancreatitis and pancreatic cancer.