An 85-year-old woman with respiratory failure and positional hypoxemia
An 85-year-old woman was brought to our intensive care unit because of worsening hypoxemia over the past day. About 3 weeks earlier she had been diagnosed with acute bilateral pulmonary emboli in the distal branches of the left and right lower lobes and right middle lobe, for which she was receiving anticoagulation therapy.
At presentation she had generalized fatigue and dyspnea at rest that was worse with exertion, but she denied having fever, chest pain, or cough. Her medical history included hypertension, hyperlipidemia, hypothyroidism, stage 1 breast cancer in remission, thromboembolic stroke, and myasthenia gravis. Before her hospital admission, she had been taking rosuvastatin, metoprolol tartrate, pyridostigmine, prednisone, furosemide, levothyroxine, and rivaroxaban. She did not smoke, she was retired, and she had not traveled recently.
Her blood pressure was 135/66 mm Hg, pulse 73 beats per minute, respiratory rate 16, temperature 35.4ºC (95.7ºF), and oxygen saturation 88% while receiving oxygen at 6 L/min via nasal cannula. Physical examination revealed mild edema in the lower extremities and basilar decreased breath sounds. She had no finger clubbing or cyanosis and was not using accessory muscles to breathe. Of note, her oxygen saturation remained more than 93% when she was recumbent but sharply dropped to less than 85% when she was upright.
Laboratory values
Results of initial laboratory testing were as follows:
- Sodium 138 mmol/L (reference range 132–148)
- Potassium 4.2 mmol/L (3.5–5.0)
- Chloride 99 mmol/L (98–111)
- Bicarbonate 29 mmol/L (23–32)
- Creatinine 0.52 mg/dL (0.7–1.4).
- White blood cell count 11.06 × 109/L (3.7–11.0)
- Hemoglobin 12.6 g/dL (12–16)
- Platelet count 211 × 109/L (150–400).
- International normalized ratio 1.4.
Electrocardiography and imaging studies
Standard 12-lead electrocardiography showed normal sinus rhythm with left axis deviation and left ventricular hypertrophy.
Chest radiography showed bilateral interstitial opacities and small pleural effusions.
Computed tomography (CT) of the chest with contrast, compared with a CT scan done 20 days earlier, showed that the pulmonary emboli had resolved.
Arterial blood gases
In view of her positional hypoxemia, blood for arterial blood gas measurements was drawn in the supine and upright positions.
Supine, with a fraction of inspired oxygen (Fio2) via high-flow nasal cannula of 45%, her values were:
- pH 7.45 (reference range 7.35–7.45)
- Pco2 34 mm Hg (36–46)
- Po2 81 mm Hg (85–95)
- Bicarbonate 23 mmol/L (22–26).
Upright, her hypoxemia was significantly worse:
- pH 7.46
- Pco2 33 mm Hg
- Po2 57 mm Hg
- Bicarbonate 23 mmol/L.
The methemoglobin level was normal on both measurements.
During her stay in the intensive care unit, she required up to 100% Fio2 because of persistent hypoxemia.
CAUSES OF HYPOXEMIA
1. So far, the patient’s laboratory tests and imaging studies point to which of the following as the most likely cause of her severe hypoxemia?
- Ventilation-perfusion (V/Q) mismatch
- Diffusion abnormality
- Hypoventilation
- Shunting
- None of the above
The arterial blood gas measurements suggested the possibility of shunting as the cause, although further imaging would be needed to confirm that.
V/Q mismatch can occur in respiratory failure due to pulmonary embolism, pulmonary edema, or shunting. If ventilation is preserved but perfusion is impaired, the V/Q ratio approaches infinity (dead-space ventilation), a situation that can be seen in pulmonary embolism. If perfusion is preserved and ventilation is impaired, the V/Q ratio approaches zero, which is consistent with a physiologic shunt.
Hypoxemia may improve in less severe forms of V/Q mismatch. In our patient, the repeat CT with contrast showed that her pulmonary embolism had resolved, so this is probably not the cause of her severe hypoxemia.
Diffusion abnormalities are due to defects in the lung parenchyma, such as in chronic obstructive pulmonary disease, interstitial lung disease, and lung fibrosis.
Hypoxemia from diffusion defects is usually aggravated by a precipitating factor that increases oxygen demand, and it usually improves with oxygen supplementation. This is unlikely in our patient, as she did not have a history of chronic interstitial lung disease and CT showed no evidence of severe lung parenchymal disease.
Hypoventilation is usually due to drugs that cause respiratory depression, to stroke, or to neuromuscular diseases such as myasthenia gravis that can cause respiratory muscle weakness. It results in elevation of Pco2 and, if not corrected, respiratory acidosis.
Our patient had a diagnosis of myasthenia gravis, though hypoventilation is unlikely in her case because she had a normal respiratory rate and low Pco2 values.
Shunting can be physiologic or anatomic and can occur in the heart or the lungs. In physiologic shunting, severe V/Q mismatch can occur when ventilation is affected, as in severe pulmonary edema and pneumonia. In anatomic shunting, a defect such as an atrial septal defect or a pulmonary arteriovenous malformation allows blood to bypass areas of ventilation from the venous to the arterial circulation, preventing it from being oxygenated. In true anatomic shunting, supplemental oxygen with 100% Fio2 has little effect, whereas in V/Q mismatch it can raise the arterial oxygen saturation.
Our patient’s radiograph did not suggest severe pneumonia or pulmonary edema, which makes these unlikely causes of her hypoxemia. At this point, because of her positional hypoxemia, further evaluation with contrast-enhanced echocardiography was needed to evaluate for anatomic shunting in the heart or lungs.
FURTHER TESTING
Transthoracic echocardiography (TTE) with agitated saline with a Valsalva maneuver was performed. Normally, no bubbles are seen in the left-sided chambers after intravenous injection of agitated saline contrast, whereas bubbles on the left side suggest an intracardiac or intrapulmonary shunt. In our patient, this test was negative, and her right ventricular systolic pressure was normal.
2. What further testing should be considered to evaluate our patient’s hypoxemia?
- High-resolution chest CT
- Transesophageal echocardiography (TEE)
- Pulmonary function testing
- Repeated arterial blood gas measurement
- Edrophonium testing
Repeat imaging with high-resolution CT would likely not provide additional information and would expose the patient to additional radiation without adding much clinical benefit.
TEE could help further evaluate the intracardiac anatomy and look for shunting, which may be missed on TTE because of suboptimal positioning or image quality.
Pulmonary function testing is useful in establishing the baseline function and impairment in respiratory volumes. If an acute myasthenic crisis is suspected, measuring the negative inspiratory force and the forced vital capacity can be useful in monitoring worsening respiratory muscle weakness and assessing the need for mechanical ventilation.
In our patient, it is unlikely that pulmonary function testing would help, since her acute respiratory failure was probably not caused by neuromuscular weakness.
Repeated arterial blood gas measurement would likely only confirm that the patient still has positional hypoxemia but would not help sort through the differential diagnosis.
Edrophonium testing is useful in diagnosing myasthenia gravis and differentiating it from other neuromuscular diseases, such as Lambert-Eaton syndrome. Edrophonium, a reversible acetylcholinesterase inhibitor, prevents degradation of acetylcholine and prolongs its effect at the synaptic cleft, thus improving muscle weakness.
Our patient has already been diagnosed with myasthenia gravis, so this test is not likely to uncover the cause of her hypoxemia.
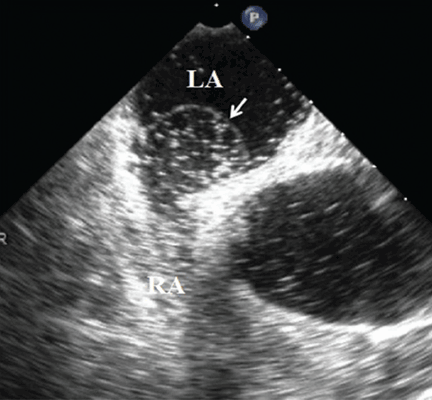
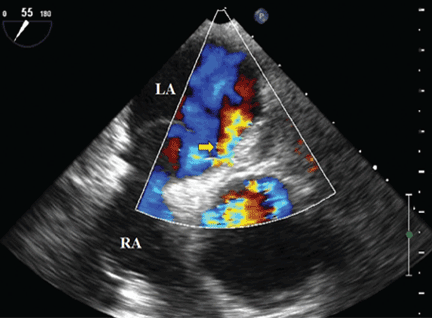
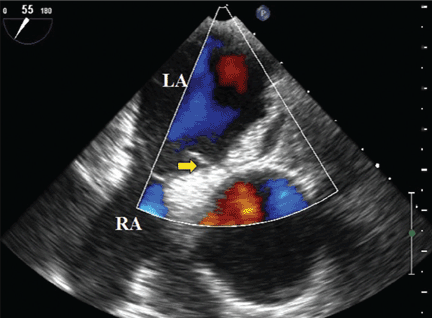
Because we still strongly suspected a shunt, TEE was performed with intravenous injection of agitated saline. TEE with the patient upright revealed intracardiac right-to-left shunting through a patent foramen ovale. The midesophageal view after saline injection showed a large interatrial septal aneurysm with total excursion of 2 cm, and right-to-left shunting within the first beat, consistent with an intracardiac shunt (Figure 1). Color Doppler imaging (Figure 2) demonstrated turbulent flow through the patent foramen ovale, consistent with right-to-left shunting, and also showed the patent foramen ovale in a closed position (Figure 3).
3. Which is now most likely the cause of our patient’s hypoxemia?
- Chronic thromboembolic pulmonary hypertension
- Myasthenic crisis
- Platypnea-orthodeoxia syndrome due to the patent foramen ovale
- Methemoglobinemia
Chronic thromboembolic pulmonary hypertension is usually a long-term result of untreated or inadequately treated thromboembolic disease (eg, pulmonary emboli), which causes vascular remodeling and pulmonary arteriopathy, which in turn leads to increased pulmonary vascular resistance and pulmonary hypertension.
This is unlikely the cause of our patient’s acute hypoxemia, as her symptoms did not suggest it. Moreover, an elevated right ventricular systolic pressure on TTE would suggest pulmonary hypertension, but TTE did not show this, and repeat chest CT indicated that her pulmonary embolism had been adequately treated and had resolved. A V/Q scan and right heart catheterization would help rule out chronic thromboembolic pulmonary hypertension, although these were not done in our patient.
Myasthenic crisis is the progressive fatiguing and paralysis of respiratory muscles ultimately requiring mechanical ventilation to sustain life. It is often brought on by infection or drug therapy.
Our patient did not require intubation and she had no signs or symptoms of myasthenic crisis such as ptosis, dysphagia, or dysarthria. She had a negative inspiratory force of −21 cm H2O, and pulmonary function testing 4 days before her hospital admission had shown a forced vital capacity of 1.84 L, making myasthenic crisis an unlikely cause of her respiratory failure.
Platypnea-orthodeoxia syndrome is a syndrome of dyspnea (platypnea) and hypoxemia (orthodeoxia) that is induced by sitting upright or standing and resolves when lying down. It is a result of right-to-left intracardiac or intrapulmonary shunting in the presence of an anatomic defect and a functional element causing redirection of shunt flow through the anatomic defect in an upright position.1 It is associated with specific cardiac, pulmonary, and hepatic diseases, such as atrial septal defect, pulmonary arteriovenous malformation, and hepatopulmonary syndrome.2 It can occur even if right-sided chamber pressures are normal, and several mechanisms of the underlying pathophysiology have been described.3
Platypnea-orthodeoxia syndrome can be triggered by an event that causes a spontaneous transient elevation of right atrial pressure and pulmonary hypertension, such as our patient’s acute pulmonary embolism. Increased right-to-left shunting occurs in an upright position, causing preferential redirection of flow from the inferior vena cava through the interatrial septum and the patent foramen ovale.4
Our patient was elderly and, like one in every four people in the world, she had had a patent foramen ovale since the day she was born. Never causing a problem, it had remained undiagnosed until complicated by platypnea-orthodeoxia syndrome after her recent pulmonary embolism.
Methemoglobinemia. Methemoglobin has a lower affinity for oxygen than normal hemoglobin. Elevations usually occur with medications such as anesthetics and nitrates and can be diagnosed through an elevated level on arterial blood gas testing.
Our patient did not have elevated methemoglobin on her blood gas measurements on admission; therefore, this is unlikely to be the diagnosis.






