Prescribing opioids in primary care: Safely starting, monitoring, and stopping
ABSTRACTChronic noncancer pain is common and often managed in the outpatient setting with chronic opioid therapy, even though the efficacy of this approach is uncertain and adverse effects are common. Some patients report meaningful benefit from opioids, but prescription drug abuse has reached epidemic proportions, and many suffer harm from opioid misuse, abuse, and diversion. Primary care providers and their care teams often struggle to balance these risks and benefits with little outside support. The authors review common challenges when starting, monitoring, and discontinuing opioids, and offer strategies for risk-reduction and patient communication.
KEY POINTS
- Predicting which patients will benefit and which ones will be harmed is difficult. We generally recommend a conservative approach to starting opioid treatment.
- Providers must periodically reassess the safety and efficacy of opioid therapy to be sure it is still indicated.
- Monitoring should be transparent and consistent. By framing monitoring in terms of safety and employing it universally, providers can minimize miscommunication and accidental stigmatization.
- When opioids are no longer safe or effective, they should be stopped. The decision can be difficult for the patient and the provider.
Chronic pain affects an estimated 100 million Americans, at a cost of $635 billion each year in medical expenses, lost wages, and reduced productivity.1 It is often managed in primary care settings with opioids by clinicians who have little or no formal training in pain management.2,3 Some primary care providers may seek assistance from board-certified pain specialists, but with only four such experts for every 100,000 patients with chronic pain, primary care providers are typically on their own.4
Although opioids may help in some chronic pain syndromes, they also carry the risk of serious harm, including unintentional overdose and death. In 2009, unintentional drug overdoses, most commonly with opioids, surpassed motor vehicle accidents as the leading cause of accidental death in the United States.5 Additionally, nonmedical use of prescription drugs is the third most common category of drug abuse, after marijuana and alcohol.6
Unfortunately, clinicians cannot accurately predict future medication misuse.7 And while the potential harms of opioids are many, the long-term benefits are questionable.8,9
For these reasons, providers need to understand the indications for and potential benefits of opioids, as well as the potential harms and how to monitor their safe use. Also important to know is how and when to discontinue opioids while preserving the therapeutic relationship.
This paper offers practical strategies to primary care providers and their care teams on how to safely initiate, monitor, and discontinue chronic opioid therapy.
STARTING OPIOID THERAPY FOR CHRONIC PAIN
Guidelines recommend considering starting patients on opioid therapy when the benefits are likely to outweigh the risks, when pain is moderate to severe, and when other multimodal treatment strategies have not achieved functional goals.10 Unfortunately, few studies have examined or demonstrated long-term benefit, and those that did examine this outcome reported reduction of pain severity but did not assess functional improvement.9 Meanwhile, data are increasingly clear that long-term use increases the risk of harm, both acute (eg, overdose) and chronic (eg, osteoporosis), especially with high doses.
Tools have been developed to predict the risk of misuse,11–13 but few have been validated in primary care, where most opioids are prescribed. This limitation aside, consensus guidelines state that untreated substance use disorders, poorly controlled psychiatric disease, and erratic treatment adherence are contraindications to opioid therapy, at least until these other issues are treated.10
Faced with the benefit-harm conundrum, we recommend a generally conservative approach to opioid initiation. With long-term functional benefit questionable and toxicity relatively common, we are increasingly avoiding chronic opioid therapy in younger patients with chronic pain.
Empathize and partner with your patient
Chronic pain care can be fraught with frustration and mutual distrust between patient and provider.14 Empathy and a collaborative stance help signal to the patient that the provider has the patient’s best interest in mind,15 whether initiating or deciding not to initiate opioids.
Optimize nonopioid therapy
In light of the risks associated with chronic opioid therapy, the clinician is urged to review and optimize nonopioid therapy before starting a patient on opioid treatment, and to maintain this approach if opioid therapy is started. Whenever possible, nonopioid treatment should include disease-modifying therapy and nondrug modalities such as physical therapy.
Judicious use of adequately dosed analgesics such as acetaminophen and nonsteroidal anti-inflammatory drugs may be sufficient to achieve analgesic goals if not contraindicated, and in some patients the addition of a topical analgesic (eg, diclofenac gel, lidocaine patches), a tricyclic or serotonin-norepinephrine reuptake inhibitor antidepressant, an anticonvulsant (eg, gabapentin), or a combination of the above can effectively address underlying pain-generating mechanisms.16 As with opioids, the risks and benefits of nonopioid pharmacotherapy should be reviewed both at initiation and periodically thereafter.
Frame the opioid treatment plan as a ‘therapeutic trial’
Starting an opioid should be framed as a “therapeutic trial.” These drugs should be continued only if safe and effective, at the lowest effective dose, and as one component of a multimodal pain treatment plan. Concurrent use of nonpharmacologic therapies (eg, physical therapy, structured exercise, yoga, relaxation training, biofeedback, cognitive behavioral therapy) and rational pharmacotherapy while promoting patient self-care is the standard of pain management called for by the Institute of Medicine.1
Set functional goals
We recommend clearly defining functional goals with each patient before starting therapy. These goals should be written into the treatment plan as a way for patient and provider to evaluate the effectiveness of chronic opioid therapy. A useful mnemonic to help identify such goals is SMART, an acronym for specific, measurable, action-oriented, realistic, and time-bound. Specific goals will depend on pain severity, but examples could include being able to do grocery shopping without assistance, to play on the floor with grandchildren, or to engage in healthy exercise habits such as 20 minutes of moderately brisk walking 3 days per week.
Obtain informed consent, and document it thoroughly
Providers must communicate risks, potential benefits, and safe medication-taking practices, including how to safely store and dispose of unused opioids, and document this conversation clearly in the medical record. From a medicolegal perspective, if it wasn’t documented, it did not happen.17
Informed consent can be further advanced with the use of a controlled substance agreement that outlines the treatment plan as well as potential risks, benefits, and practice policies in a structured way. Most states now either recommend or mandate the use of such agreements.18
Controlled substance agreements give providers a greater sense of mastery and comfort when prescribing opioids,19 but they have important limitations. In particular, there is a lack of consensus on what the agreement should say and relatively weak evidence that these agreements are efficacious. Additionally, a poorly written agreement can be stigmatizing and can erode trust.20 However, we believe that when the agreement is written in an appropriate framework of safety at an appropriate level of health literacy and with a focus on shared decision-making, it can be very helpful and should be used.
Employ safe, rational pharmacotherapy
Considerations when choosing an opioid include its potency, onset of action, and half-life. Comorbid conditions (eg, advanced age,21 sleep-disordered breathing22) and concurrent medications (eg, benzodiazepines, anticonvulsants, muscle relaxants) also affect decisions about the formulation, starting dose, rapidity of titration, and ceiling dose. Risk of harm increases in patients with such comorbid factors, and it is prudent to start with lower doses of shorter-acting medications until patients can demonstrate safe use. Risk of unintentional overdose is higher with higher prescribed doses.23 Pharmacologically there is no analgesic dose ceiling, but we urge caution, particularly in opioid-naive patients.
A patient’s response to any particular opioid is idiosyncratic and variable. There are more than 100 known polymorphisms in the human opioid mu-receptor gene, and thus differences in receptor affinity and activation as well as in metabolism make it difficult to predict which opioid will work best for a particular patient.24 However, a less potent opioid receptor agonist with less addictive potential, such as tramadol or codeine, should generally be tried first before escalating to a riskier, more potent opioid such as hydrocodone, oxycodone, or morphine. This “analgesic ladder,” a concept introduced by the World Health Organization in 1986 to provide a framework for managing cancer pain, has been adapted to a variety of chronic pain syndromes.25
Methadone deserves special mention. A strongly lipophilic molecule with a long and variable half-life, it accumulates in fat,26 and long after the analgesic effect has worn off, methadone will still be present. Repeated dosing or rapid dose escalation in an attempt to achieve adequate analgesia may result in inadvertent overdose. Additionally, methadone can prolong the QT interval, and periodic electrocardiographic monitoring is recommended.27 For these reasons, we recommend avoiding the use of methadone in most cases unless the provider has significant experience, expertise, or support in the safe use of this medication.
Table 1 summarizes these recommendations.
MONITORING AND SAFETY
Providers must periodically reassess the safety and efficacy of chronic opioid therapy to be sure that it is still indicated.10 Since we cannot accurately predict which patients will suffer adverse reactions or demonstrate aberrant behaviors,7 it is important to be transparent and consistent with monitoring practices for all patients on chronic opioid therapy.17 By framing monitoring in terms of safety and employing it universally, providers can minimize miscommunication and accidental stigmatization.
Prescription monitoring programs
In 2002, Congress appropriated funding to the US Department of Justice to support prescription monitoring programs nationally.28 At the time of this writing, Missouri is the only state without an approved monitoring program.29
Although the design and function of the programs vary from state to state, they require pharmacies to collect and report data on controlled substances for individual patients and prescribers. These data are sometimes shared across state lines, and the programs enhance the capacity of regulatory and law enforcement agencies to analyze controlled substance use.
Prescribers can (and are sometimes required to) register for access in their state and use this resource to assess the opioid refill history of their patients. This powerful tool improves detection of “doctor-shopping” and other common scams.30
Additionally, recognizing that the risk of death from overdose increases as the total daily dose of opioids increases,23 some states provide data on their composite report expressing the morphine equivalent daily dose or daily morphine milligram equivalents of the opioids prescribed. This information is valuable to the busy clinician; at a glance the prescriber can quickly discern the total daily opioid dose and use that information to assess risk and manage change. Furthermore, some states restrict further dose escalation when the morphine equivalent daily dose exceeds a predetermined amount (typically 100 to 120 morphine milligram equivalents).
Tamper-resistant prescribing
To minimize the risk of prescription tampering, simple techniques such as writing out the number of tablets dispensed can help, and use of tamper-resistant prescription paper has been required for Medicaid recipients since 2008.31
When possible, we recommend products with abuse-deterrent properties. Although the science of abuse deterrence is relatively new and few products are labeled as such, a number of opioids are formulated to resist deformation, vaporization, dissolving, or other physical tampering. Additionally, some abuse-deterrent opioid formulations contain naloxone, which is released only when the drug is deformed in some way, thereby decreasing the user’s response to an abused substance or resulting in opioid withdrawal.32
Urine drug testing
Although complex and nuanced, guidelines recommend urine drug testing to confirm the presence or absence of prescribed and illicit substances in the body.10 There is no consensus on when or how often to test, but it should be done randomly and without forewarning to foil efforts to defeat testing such as provision of synthetic, adulterated, or substituted urine.
Providers underuse urine drug testing.33 We recommend that it be done at the start of opioid therapy, sporadically thereafter, when therapy is changed, and whenever the provider is concerned about possible aberrant drug use.
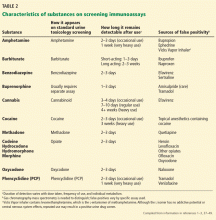
Understanding opioid metabolism, cross-reactivity, and the types of tests available will help avoid misinterpretation of results.34 For example, a positive “opiate” result in most screening immunoassay tests does not reflect oxycodone use, since tests for synthetic opioids often need to be ordered separately; the commonly used Cedia opiate assay cross-reacts with oxycodone at a concentration of 10,000 ng/mL only 3.1% of the time.35 Immunoassay screening tests are widely available, sensitive, inexpensive, and fast, but they are qualitative, have limited specificity, and are subject to false-positive and false-negative results.36 Table 2 outlines some common characteristics of substances on screening immunoassays, including reported causes of false-positive results.37–39
Confirmatory testing using gas chromatography or mass spectroscopy is more expensive and slower to process, but is highly sensitive and specific, quantitative, and useful when screening results are difficult to interpret.
Knowing how and when to order the right urine drug test and knowing how to interpret the results are skills prescribers should master.